The molar volume for a mixture of methanol (1) + water (2) at 298.15 K and 1
Question:
The molar volume for a mixture of methanol (1) + water (2) at 298.15 K and 1 bar is given as
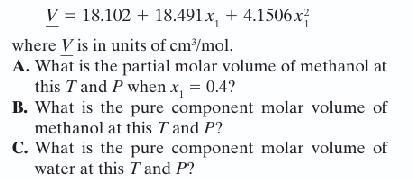
D. Let’s assume you have an equimolar mixture of water and methanol. What will be the molar vol ume of this mixture according to the functional form given in the problem? What will be the molar volume of this mixture if you assumed this mixture behaved as an ideal solution?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:





