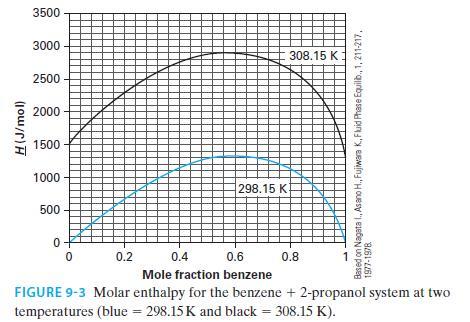
In a flow process, you mix 200 g/s of benzene at 308.15 K with 300 g/s of
Question:
In a flow process, you mix 200 g/s of benzene at 308.15 K with 300 g/s of 2-propanol at 298.15 K. Using Figure 9-3, answer the following;
A. What is the concentration of the resulting mixture?
B. Estimate the heat effect (added/removed) of this mixing if it is done such that the resulting tem perature is 298.15 K.
C. If the mixing were done in an adiabatic fashion, estimate the resulting solution temperature.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:





