The steam reforming of natural gas into syngas features the gas phase reaction: which has an equilibrium
Question:
The steam reforming of natural gas into syngas features the gas phase reaction:
![]()
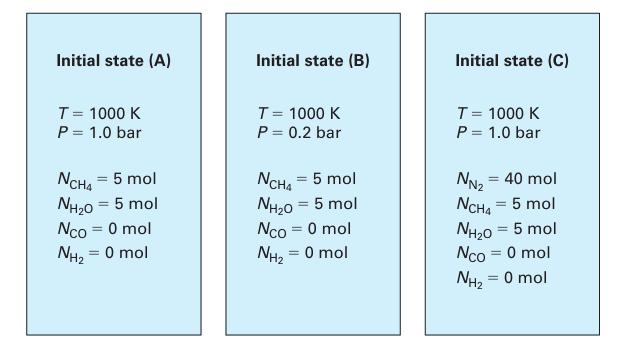
which has an equilibrium constant K1000 = 26.6 (Lu, 2010). This reaction is to be carried out in a sealed reaction vessel (Figure 14-4) that has a constant T = 1000 K and a constant pressure. The reactor initially contains 5 moles each of methane and water vapor, and no carbon monoxide or hydrogen. For each of the following cases, find the extent of reaction at equilibrium.
A. The reactor has constant P = 1.0 bar.
B. The reactor has constant P = 0.2 bar.
C. The reactor has constant P = 1.0 bar, and initially contains 40 moles of nitrogen in addition to the methane and water vapor
Figure 14‑4.
Step by Step Answer:

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco





