This problem revisits Example 15-4. Re-do the problem three times, changing the volume of the system to
Question:
This problem revisits Example 15-4. Re-do the problem three times, changing the volume of the system to the following.
A. 300 L
B. 1000 L
C. 1500 L
D. Use graphs or tables to compare the results of parts A through C to the original problem that used a volume of 600 L. Give interpretations based on physical phenomena for any trends that are evident.
Example 15-4.
The three isomers of xylene are o-xylene, m-xylene, and p-xylene (standing for “ortho,” “meta,” and “para”) shown in Figure 15-4.
Inter-conversions between these isomers can be modeled through these two chemical reactions:
(R1): o-xylene ↔ m-xylene
(R2): m-xylene ↔ p-xylene
A. 10 moles of xylene are placed in a vessel that is maintained at T = 100°C and P = 1 atm. Find the amounts of o-, p-, and m-xylene that are present at equilibrium.
B. 10 moles of xylene are placed in a vessel that is maintained at T = 100°C and has a volume of 600 L. The vessel contains both liquid and vapor. The liquid phase is exposed to a catalyst but the vapor is not; thus we can assume reactions R1 and R2 attain equilibrium in the liquid phase but do not occur at all in the vapor phase. Find the contents of the reactor at equilibrium..
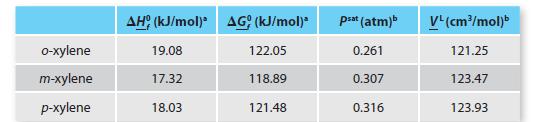
We will use the following data, which uses T = 25°C and P 5 1 atm, rather than P = 1 bar, as a reference state
Step by Step Answer:

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco





