Two moles of an ideal gas are confined in a piston-cylinder device, initially at P = 5
Question:
Two moles of an ideal gas are confined in a piston-cylinder device, initially at P = 5 bar and T = 300 K. The atmosphere is at P = 1 bar but there is a pile of sand on top of the piston, as shown in Figure 4-3. The mass of the sand is sufficient to make the total downward pressure on the piston 5 bar. The grains of sand are removed one at a time, leading to a gradual decrease in pressure and a gradual expansion of the gas, until the gas is at P = 1 bar. The temperature of the gas is T = 300 K throughout.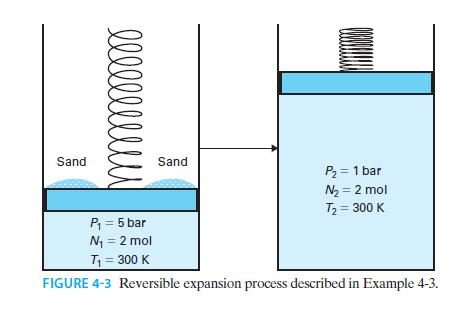
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:





