Using the NIST Webbook, if one looks up the molar enthalpy of pure benzene at 308.15 K
Question:
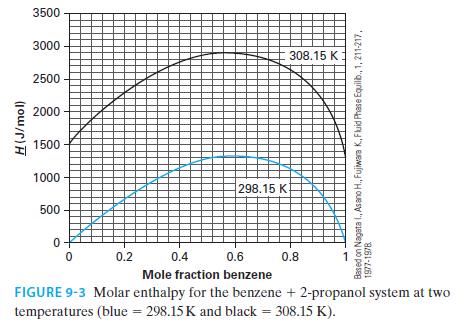
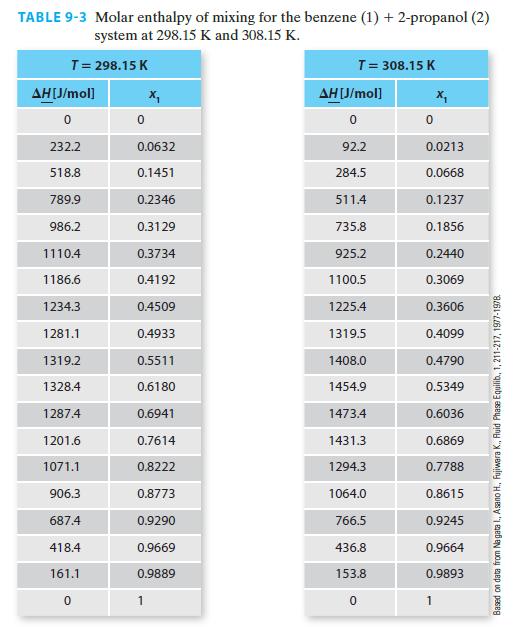
Using the NIST Webbook, if one looks up the molar enthalpy of pure benzene at 308.15 K and 1 bar, the reported value is –6359.6 J/mol. Figure 9-3 has this same property as being equal to 1356.9 J/mol (via Example 9-2). Whose value, if any, is in error? Please explain your answer.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:





