You are interested in modeling the vapor-liquid equilibrium of the propane (1) + n-pentane (2) system at
Question:
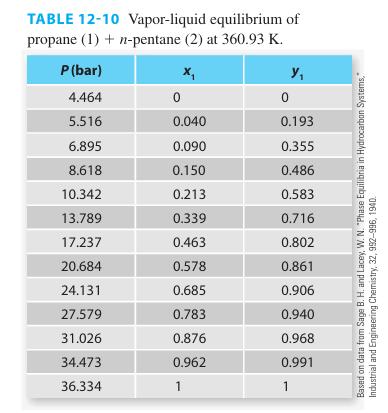
You are interested in modeling the vapor-liquid equilibrium of the propane (1) + n-pentane (2) system at 360.93 K. You know that the vapor pressure of propane at this temperature is 36.334 bar while the vapor pressure of the n-pentane is 4.464 bar. Since the components in the mixture are relatively similar in size and interactions, you want to use the ideal solution model. However, the mixture pressure will be well above that needed to comfortably use the ideal gas model. Thus, Raoult’s Law is not the preferred approach. Create a Pxy plot showing the behavior of this system if modeled as an ideal solution for both the liquid and the vapor phases. Compare your model results with both the Raoult’s Law predictions and the experimental data given in Table 12-10
Step by Step Answer:

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco




