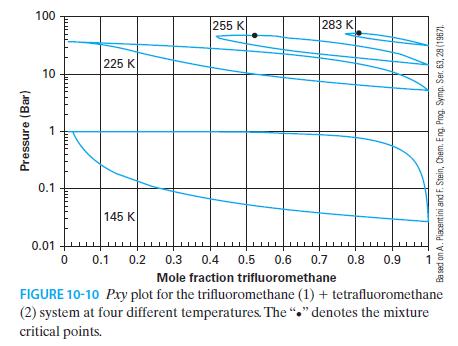
You have 80 moles of trifluoromethane and 20 moles of tetrafluoromethane at 10 bar and 225 K.
Question:
You have 80 moles of trifluoromethane and 20 moles of tetrafluoromethane at 10 bar and 225 K. Using Figure 10-10 and the lever rule, determine the number of moles and concentration of the liquid and vapor phases that are in equilibrium.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:





