A counter-current packed tower will be used to remove H 2 S vapor from a N 2
Question:
A counter-current packed tower will be used to remove H2S vapor from a N2-rich gas stream. In the present process, the inlet gas stream entering the bottom of the tower contains 5.0 mole% H2S at a superficial molar flowrate of 10 kgmole/m2/h, and the processed gas stream exiting the top of the tower must contain no more than 1.0 mole% H2S. Pure solvent containing no H2S enters the top of the tower. The total molar concentration of the solvent (CL) is 50 kgmole/m3.
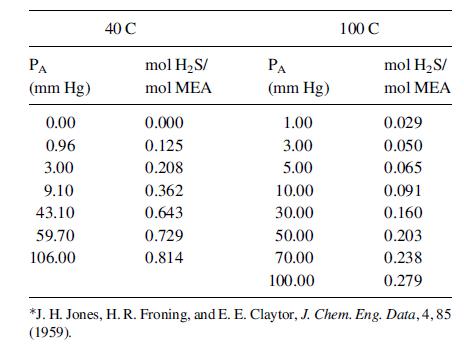
The equilibrium distribution of H2S between the gas and liquid at process temperature of 40 and 100 C, provided by Jones et al.*, is provided in the table. The total system pressure is 1.0 atm.
a. What is the superficial molar flowrate of the gas stream exiting the top of the tower?
b. What is the minimum solvent flowrate for the process? Compare results at 40 and 100 C and comment on the differences.
Step by Step Answer:

Fundamentals Of Momentum Heat And Mass Transfer
ISBN: 9781119723547
7th Edition
Authors: James Welty, Gregory L. Rorrer, David G. Foster





