As society searches for technical solutions to global warming, vast ponds of photosynthetic algae are seen as
Question:
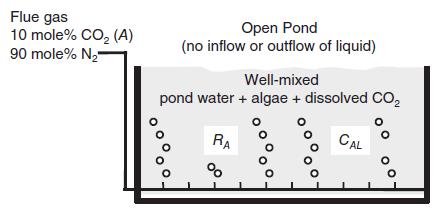
As society searches for technical solutions to global warming, vast ponds of photosynthetic algae are seen as one possible solution for the removal of CO2 from combustion of fossil fuels. Consider the process shown in the figure (next page), where a flue gas containing a binary mixture 10 mole% CO2 and 90 mole% N2 is bubbled into a pond containing photosynthetic, single-celled algae that are uniformly suspended in the liquid water. The agitation provided by the rising bubbles keeps the liquid suspension well mixed. The algae consume dissolved CO2 according to a first-order reaction of the form
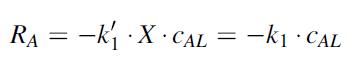
where RA is the CO2 consumption per unit volume of liquid suspension (gmole CO2/m3 s, k1 is the rate constant for CO2 uptake by the algal cells (m3/g cells/h), X is the algal cell density (g cells/m3), cAL is the concentration of dissolved CO2 (gmole CO2/m3), and k1 is the apparent rate constant for CO2 uptake (k1 k1 X h–1). In the present process, there is no inflow or outflow of water into the pond. However, there is a constant delivery of CO2 to the liquid suspension by the bubbled-in flue gas, and a constant consumption of dissolved CO2 by the photosynthetic algae, with k1 = 0 06435 m3/g cells h. Furthermore, since CO2 is not very soluble in water, it was found that the composition of CO2 gas inside the gas bubble decreased only slightly as it traveled up the water column. Therefore, the partial pressure of CO2 gas inside the gas bubble (pA) is considered constant. However, there is still a significant rate of transfer of CO2 into the liquid phase. The open pond is maintained at 20 C. At 20 C, the Henry’s law constant for the dissolution of CO2 gas in the liquid is 0.025 atm m3/gmole, the mass density of liquid is 1000 kg/m3, and the viscosity of the liquid is 993 x 10–6 kg/m s. The aeration system is designed so that the average bubble size is 7.0 mm and the desired interphase area/liquid volume is a 5.0 m2/m3. The mass density of the gas mixture is 1.2 kg/m3. The total liquid volume of the pond is kept constant.
a. What is the molecular diffusion coefficient of CO2 in water?
b. What is the mass-transfer coefficient for CO2 on the liquid film side of the gas bubble, kL?
c. Develop a material balance model for predicting the dissolved CO2 concentration in the pond water. The model must be in algebraic form and contain the following variables: cAL, pA, H, kL, a, k1.
d. If the cell density in the pond is maintained at 50 g cells/m3,what is the predicted concentration of dissolved CO2 in the pond, cAL?
e. If the total pond volume is 1000 m3, what is the total CO2 removal rate in kg CO2/h?
Step by Step Answer:

Fundamentals Of Momentum Heat And Mass Transfer
ISBN: 9781119723547
7th Edition
Authors: James Welty, Gregory L. Rorrer, David G. Foster





