A 10.0 g sample of liquid water is sealed in a 1515 mL flask and allowed to
Question:
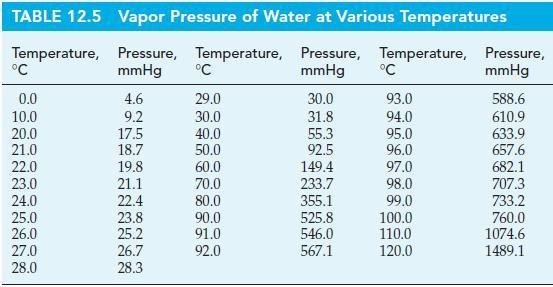
A 10.0 g sample of liquid water is sealed in a 1515 mL flask and allowed to come to equilibrium with its vapor at 27 °C. What is the mass of H2O(g) present when equilibrium is established? Use vapor pressure data from Table 12.5.
Table 12.5
Transcribed Image Text:
TABLE 12.5 Temperature, °C 0.0 10.0 20.0 21.0 22.0 23.0 24.0 25.0 26.0 27.0 28.0 Vapor Pressure of Water at Various Temperatures Pressure, Temperature, Pressure, Temperature, mmHg mmHg 4.6 9.2 17.5 18.7 19.8 21.1 22.4 23.8 25.2 26.7 28.3 °C 29.0 30.0 40.0 50.0 60.0 70.0 80.0 90.0 91.0 92.0 30.0 31.8 55.3 92.5 149.4 233.7 355.1 525.8 546.0 567.1 °C 93.0 94.0 95.0 96.0 97.0 98.0 99.0 100.0 110.0 120.0 Pressure, mmHg 588.6 610.9 633.9 657.6 682.1 707.3 733.2 760.0 1074.6 1489.1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
Find the vapor pressure of water at 27 C From Table 125 the vapor press...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
What will be the profit percentage after selling an article at certain price if there is a loss of 40 percent when the same article is sold at 2/5 of the earlier selling price?
-
Find given En+1 10 = 3 n = and = 4.
-
Which of the following are the government inspectors whose mission is to reduce Medicare improper payments through the detection and collection of overpayments, identification of underpayments, and...
-
In 1976, Mohamed EI-Iladad earned an undergraduate accounting degree in his native Egypt. Before he began his accounting career, El-Hadad completed his compulsory service in the Egyptian military...
-
Plaintiff brings this cause of action against a manufacturer for the loss of one leg below the hip. The leg was lost when caught in the gears of a screw auger machine sold and installed by the...
-
Identify the reagents a?c in the following scheme: CH C -CH CH H -CH H.
-
In the benzene adsorber of Example 9.7, the flow rate is increased to \(0.25 \mathrm{~m}^{3} / \mathrm{s}\). Calculate the breakthrough time and the fraction of the bed adsorption capacity that has...
-
Describe in general terms, how you think the distribution system, for McDonalds works.
-
1. Why have neo-classical economists generally argued that international economic relations are not zero-sum in character? What theoretical frameworks have they used to support this argument? It's...
-
Some vapor pressure data for Freon-12, CCl 2 F 2 , once a common refrigerant, are -12.2 C, 2.0 atm; 16.1 C, 5.0 atm; 42.4 C, 10.0 atm; 74.0 C, 20.0 atm. Also, bp = -29.8 C, T c = 111.5 C, P c = 39.6...
-
A 7.53 L sample of N 2 (g) at 742 mmHg and 45.0 C is bubbled through CCl 4 (l) at 45.0 C. Assuming the gas becomes saturated with CCl 4 (g) what is the volume of the resulting gaseous mixture if the...
-
True or False: When conducting an economic analysis, an engineer should consider costs from one and only one cost viewpoint.
-
20 40 Output L* K* LTC LAC LMC 0 25 20 800 50 15 16 75 33 20 20 100 31 2 300 125 78 150 66 175 140 44 32 6 440 The price of labour is $20 per unit and the price of capital is $40 per unit. To produce...
-
In today's business world, communication channels are increasing rapidly, and the demand for connectivity between employees and organizational leaders is also growing, which has further heightened...
-
How does Amazon's communication system support upward, downward, and horizontal communication? How does Amazon's communication system reflect the implementation of the interpersonal communication...
-
Please provide critique of the article "Using Real-World Examples to Enhance the Relevance of theIntroductory Statistics Course" by Friedman, Friedman and Amoo. Link...
-
Define and describe the processes of project communication management according to the extract above. Describe the meaning of communication to implemented projects and what the project managers must...
-
At the beginning of its fiscal year, Lakeside Inc. leased office space to LTT Corporation under a ten-year lease agreement. The contract calls for quarterly lease payments of $25,000 at the end of...
-
CdF2 (s) Cd+ (aq) + 2 F- (aq) 1. A saturated solution of CdF2 is prepared. The equilibrium in the solution is represented above. In the solution [Cd+] eq = 0.0585 M and [F-] eq = 0.117 M. a....
-
Dividend Growth Model Based on the dividend growth model, what are the two components of the total return on a share of stock? Which do you think is typically larger?
-
Growth Rate In the context of the dividend growth model, is it true that the growth rate in dividends and the growth rate in the price of the stock are identical?
-
Voting Rights When it comes to voting in elections, what are the differences between US political democracy and U.S. corporate democracy?
-
In 2024, the Westgate Construction Company entered into a contract to construct a road for Santa Clara County for $10,000,000. The road was completed in 2026. Information related to the contract is...
-
Given the following Year 12 balance sheet data for a footwear company: Balance Sheet Data Cash on Hand Total Current Assets Total Fixed Assets Total Assets Accounts Payable $ 10,000 150,000 250,000...
-
Blossom Corporation has a defined-benefit pension plan covering its 1460 employees. Blossom agrees to amend its pension benefits. The prior service cost associated with the amendment is $813120. The...

Study smarter with the SolutionInn App


