(A) A solution that is 20.0% ethanol, by volume, is found to have a density of 0.977...
Question:
(A) A solution that is 20.0% ethanol, by volume, is found to have a density of 0.977 g/mL. Use this fact, together with data from Example 14-1, to determine the mass percent ethanol in the solution.
(B) Ionic liquids (ILs) are salts with relatively low melting points and vapor pressures. Because of their low volatilities, chemists continue to investigate the potential of ILs as safer and “greener” solvents. 1-Butyl-3-methylimidazolium hexafluorophosphate, [BMIM][PF6], is a viscous, colorless, hydrophobic, and insoluble ionic liquid with a molar mass of 284.1 g/mol and a density of 1.38 g/mL. In a solution of [BMIM][PF6] and carbon dioxide at 8 MPa, the mole fraction of CO2 is 0.60. What is the solution concentration expressed as the
(a) Molarity of carbon dioxide and
(b) Molality of carbon dioxide? Assume that there is no volume change when CO2 is added to [BMIM][PF6].
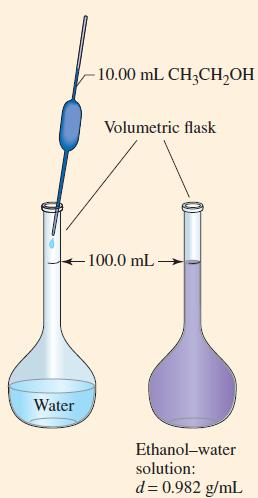
Example 14-1
An ethanol–water solution is prepared by dissolving 10.00 mL of ethanol, CH3CH2OH (d = 0.789 g/mL), in a sufficient volume of water to produce 100.0 mL of a solution with a density of 0.982 g/mL (Fig. 14-1). What is the concentration of ethanol in this solution expressed as
Figure 14-1
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette





