(A) Compound A with the formula C 3 H 8 O is soluble in water and reacts...
Question:
(A) Compound A with the formula C3H8O is soluble in water and reacts with sodium metal, producing bubbles of gas. When compound A is treated with chromic acid (a mixture of Na2Cr2O7 and H2SO4), compound B is formed. Compound B dissolves readily in Na2CO3(aq) and reacts with ethanol, yielding compound C which has a fruity fragrance. Identify compounds A, B, and C.
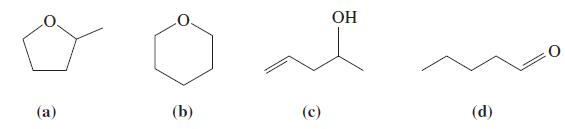
(B) The following molecules all have the molecular formula C5H10O. You suspect that you have a sample of one of these compounds. What tests could you perform to ascertain which of these compounds you have?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





