Question: A method for estimating electron affinities is to extrapolate Z eff values for atoms and ions that contain the same number of electrons as the
A method for estimating electron affinities is to extrapolate Zeff values for atoms and ions that contain the same number of electrons as the negative ion of interest. Use the data in the table to answer the questions that follow.
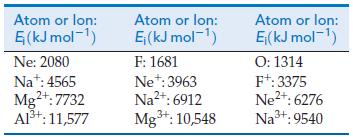
(a) Estimate the electron affinity of F, and compare it with the experimental value.
(b) Estimate the electron affinities of O and N.
(c) Examine your results in terms of penetration and screening.
Atom or lon: (kJ mol-) Ne: 2080 Na: 4565 Mg+: 7732 Al+: 11,577 Atom or lon: E (kJ mol-) F: 1681 Ne+: 3963 Na+: 6912 Mg+: 10,548 Atom or lon: E(kJ mol-) O: 1314 F+: 3375 Ne+: 6276 Na+: 9540
Step by Step Solution
3.37 Rating (153 Votes )
There are 3 Steps involved in it
a Estimate the electron affinity of F and compare it with the experimental value To estimate the electron affinity of F we can extrapolate the Zeff va... View full answer

Get step-by-step solutions from verified subject matter experts


