A mixture of H 2 S(g) and CH 4 (g) in the mole ratio 2 : 1
Question:
A mixture of H2S(g) and CH4(g) in the mole ratio 2 : 1 was brought to equilibrium at 700 °C and a total pressure of 1 atm. On analysis, the equilibrium mixture was found to contain 9.54 x 10-3 mol H2S. The CS2 present at equilibrium was converted successively to H2SO4 and then to BaSO4 1.42 x 10-3 mol BaSO4; was obtained. Use these data to determine Kp at 700 °C for the reaction below. Assume pressures are expressed in atmospheres.
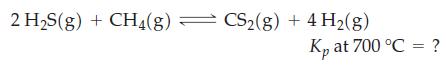
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





