Show that in terms of mole fractions of gases and total gas pressure the equilibrium constant expression
Question:
Show that in terms of mole fractions of gases and total gas pressure the equilibrium constant expression for
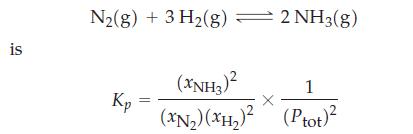
Transcribed Image Text:
is N₂(g) + 3 H₂(g) = 2 NH3(g) Kp (XNH3)² 1 (XN₂) (XH₂)² (Ptot)²
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
The equilibrium constant expression for a gasphase reaction can be written in terms of either partia...View the full answer

Answered By

Sinmon Warui Kamau
After moving up and down looking for a job, a friend introduced me to freelance writing. I started with content writing and later navigated to academic writing. I love writing because apart from making a living out of it, it is also a method of learning and helping others to learn.
5.00+
40+ Reviews
45+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Methane (CH 4 ) gas flows into a combustion chamber at a rate of 200. L/ min at 1.50 atm and ambient temperature. Air is added to the chamber at 1.00 atm and the same temperature, and the gases are...
-
A continuous distillation unit, consisting of a perforated-tray column together with a partial reboiler and a total condenser, is to be designed to operate at atmospheric pressure to separate ethanol...
-
Write the equilibrium constant expression for the dissolution of ammonia in water: Use this equilibrium constant expression to estimate the partial pressure of NH 3 (g) over a solution containing 5 x...
-
Olmsted Co. has small computer chips assembled in Poland and transports the final assembled products to the parent, where they are sold by the parent in the U.S. The assembled products are invoiced...
-
Columbia Paper has the following stockholders equity account. The firms common stock has a current market price of $30 per share. Preferred stock .............$100,000 Common stock (10,000 shares at...
-
Brabant NV of the Netherlands is a wholesale distributor of Dutch cheeses that it sells throughout the European Community. Unfortunately, the company's profits have been declining, which has caused...
-
Ormet Primary Aluminum Corporation, operated an aluminum smelter plant in Hannibal, Ohio. The facility ceased production in October 2013 in order to liquidate its assets after filing for bankruptcy...
-
On May 1, 2017, Herron Corp. issued $600,000, 9%, 5-year bonds at face value. The bonds were dated May 1, 2017 and pay interest annually on May 1. Financial statements are prepared annually on...
-
1.Libby just expanded her restaurant. She projects revenue will reach $35,000 for the new restaurant in the first year and increase by 25% over the next three years. Expenses are 75% of sales. The...
-
A mixture of H 2 S(g) and CH 4 (g) in the mole ratio 2 : 1 was brought to equilibrium at 700 C and a total pressure of 1 atm. On analysis, the equilibrium mixture was found to contain 9.54 x 10 -3...
-
What is the apparent molar mass of the gaseous mixture that results when COCl 2 (g) is allowed to dissociate at 395 C and a total pressure of 3.00 atm? Think of the apparent molar mass as the molar...
-
The University of Medford pays the full tuition for the children of faculty members at any university in the world. Recently, this policy has received bad publicity. The argument has been made that...
-
If company A is sending money to Company B, then what is the disbursement float for Company A?
-
A spherical buoy of diameter 0.6m and mass 30kg is attached to the seabed by mooring rope and floats fully submerged. determine the tension in the mooring rope. the density of sea water is 1020kgm^-3.
-
5. For the 0-1 Knapsack Problem, the item #1 has weight of 4 and the value of $12; the item #2 has weight of 1 and the value of $1; the item #3 has weight of 5 and the value of $10; and the item #4...
-
Let = {0, 1} and consider the state-transition diagram given in Figure 1. 0 1 A 0 0 B D 1 0 Figure 1: State-transition diagram for Question 4. (a) Give examples of three strings that are accepted by...
-
What is the process involved in cleaving plasmid DNA using restriction enzymes, and how does this method contribute to molecular biology research and genetic engineering endeavors?
-
Calculate the following: a. The first year of depreciation on a residential rental building costing 200,000 purchased on May 2, 2012. b. The second year of depreciation on a computer costing 3,000...
-
Organizations are increasing their use of personality tests to screen job applicants. What are some of the advantages and disadvantages of this approach? What can managers do to avoid some of the...
-
Washington Co. operates a chain of bookstores. The company maintains a defined contribution pension plan for its employees. The plan requires quarterly installments to be paid to the funding agent,...
-
In a recent years financial statements, Procter & Gamble showed an unfunded pension liability of $2,637 million and a periodic pension cost of $183 million. Explain the meaning of the $2,637 million...
-
Lachgar Industries warrants its products for one year. The estimated product warranty is 4% of sales. Assume that sales were $210,000 for June. In July, a customer received warranty repairs requiring...
-
Identify a manager who is highly concerned for production and uses rewards to maintain the loyalty of employees?
-
Write a response to I have enjoy learning every aspect of this course. I really enjoy working on DocuCare. It has really help me improved my charting at work. It also have understand the important of...
-
We also want to place wet floor signs when mopping and have our staff use the other side of the walkway for egress during this time to ensure they are not walking on wet floors. How would we ensure...

Study smarter with the SolutionInn App


