A possible reaction for converting methanol to ethanol is (a) Calculate r H r S,
Question:
A possible reaction for converting methanol to ethanol is
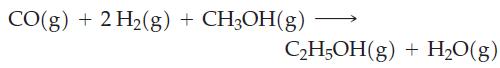
(a) Calculate ΔrH° ΔrS°, and ΔrG° for this reaction at 25 °C.
(b) Is this reaction thermodynamically favored at high or low temperatures? At high or low pressures? Explain.
(c) Estimate K for the reaction at 750 K.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





