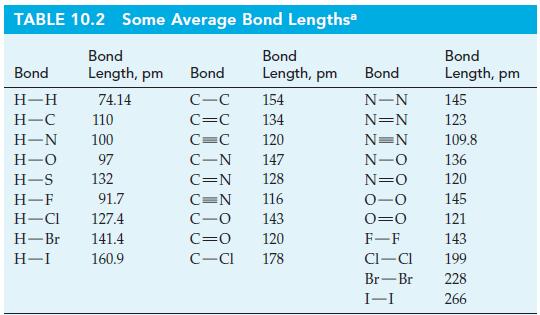
A relationship between bond lengths and single bond covalent radii of atoms. Use this relationship together with
Question:
A relationship between bond lengths and single bond covalent radii of atoms. Use this relationship together with appropriate data from Table 10.2 to estimate these single-bond lengths.
(a) I—Cl;
(b) O—Cl;
(c) C—F;
(d) C—Br.
Table 10.2
Transcribed Image Text:
TABLE 10.2 Some Average Bond Lengths Bond Bond Length, pm Length, pm Bond H-H H-C 110 H-N 100 H-O 97 H-S 132 H-F 91.7 H-Cl 127.4 H-Br 141.4 H-I 160.9 74.14 Bond C-C C=C C=C C-N C=N 154 134 120 147 128 116 143 120 C=N C-O C=0 C-Cl 178 Bond N-N N=N N=N N-O N=O 0-0 0=0 F-F CI-CI Br-Br I-I Bond Length, pm 145 123 109.8 136 120 145 121 143 199 228 266
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
The bond lengths of these bonds can be determined using the average bond lengths of the co...View the full answer

Answered By

Fahmin Arakkal
Tutoring and Contributing expert question and answers to teachers and students.
Primarily oversees the Heat and Mass Transfer contents presented on websites and blogs.
Responsible for Creating, Editing, Updating all contents related Chemical Engineering in
latex language
4.40+
8+ Reviews
22+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Use Table 8.4 to estimate the enthalpy change for each of the following reactions: a. H2C == O (g) + HCl (g) H3C - O - Cl (g) b. H2O2 (g) + 2CO (g) H2 (g) + CO2 (g) (c). 3H2C == CH2 (g) C6H12 (g)...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-6. On December 12, Irene purchased the building where her store is located. She paid...
-
A utilization greater than one suggests that the mean service time is higher than the mean inter-arrival time. True False QUESTION 3 It costs five times more money to retain a current customer than...
-
Ronald D. Johnson is a former employee of International Business Machines Corporation (IBM). As part of a downsizing effort, IBM discharged Johnson. In exchange for an enhanced severance package,...
-
Figure 2 shows a cross section through a system that consists of an infinitely long charged rod centred in a thick cylindrical shell of inner radius R and outer radius 2R. The shell is made of a...
-
T. Christian Cooper was a partner to Sanders and Richard Campbell d/b/a The Mullen Company. In 2001, Cooper helped bring about a management agreement between The Mullen Co. and Newnan Crossing...
-
Suppose selected financial data of Target and Wal-Mart for 2014 are presented here (in millions). Instructions (a) For each company, compute the following ratios. (1) Current ratio. (2) Accounts...
-
How to convert this Entity-Relationship data model into a relational database model? Contains Inventory Inventory ID Customer Cust_ID Amount Availible Cust LName Must Have Coffee_Type Cust Fname...
-
Estimate the lengths of the following bonds and indicate whether your estimate is likely to be too high or too low: (a) ICl; (b) CF.
-
Without referring to tables in the text, indicate which of the following bonds you would expect to have the greatest bond length, and give your reasons. (a) O 2 ; (b) N 2 ; (c) Br 2 ; (d) BrCl.
-
Which of the following statements is true? a. The concept of defense-in-depth reflects the fact that security involves the use of a few sophisticated technical controls. b. Information security is...
-
Determine the logging and auditing deficiencies of the current environment. Then, create official policies for the following: Logging (Think about what you want to capture and whether it is...
-
Which bio conservation strategy is used when the endangered species are removed from the unsafe or threatened habitat and placed under human care? How is this strategy different from the other...
-
What type of operating system is Unix time sharing System? Describe two major features of Unix time sharing system.
-
Discuss whether the brand portfolio of Banyan Tree, Angsana, and Cassia, as well as the product portfolio of beach resorts, services residences, city hotels, spas, galleries, and museum shops fit as...
-
In what ways is internal alignment an important policy in a strategic perspective of compensation? Discuss the factors that influence internal pay structures. based on your scholarly research, how do...
-
Search on the Internet for the 2010 annual report for Sanofi-Aventis . Find the accounts receivable disclosure note. Required: 1. Sanofi-Aventis subtracts impairment from the gross value of accounts...
-
A genetically engineered strain of Escherichia coli (E. coli) is used to synthesize human insulin for people suffering from type I diabetes mellitus. In the following simplified reaction scheme,...
-
A factory costs $400,000. You reckon that it will produce an inflow after operating costs of $100,000 in year 1, $200,000 in year 2, and $300,000 in year 3. The opportunity cost of capital is 12...
-
Halcyon Lines is considering the purchase of a new bulk carrier for $8 million. The forecasted revenues are $5 million a year and operating costs are $4 million. A major refit costing $2 million will...
-
As winner of a breakfast cereal competition, you can choose one of the following prizes: a. $100,000 now. b. $180,000 at the end of five years. c. $11,400 a year forever. d. $19,000 for each of 10...
-
5. Repeat Problem 1, except for an M/M/3 queue with mean interarrival time 1.25 minutes and mean service time 3 minutes at each of the three servers. Hint: You might want to consider creating a...
-
QUESTION 4 Consider a microprogrammed system with a control memory of 1024 words of 24 bits. The microinstruction has three fields: (1) microoperations field, (2) select field, and (3) branch address...
-
Jump to level 1 session begins SET GLOBAL TRANSACTION ISOLATION LEVEL SERIALIZABLE; session ends session begins SET TRANSACTION ISOLATION LEVEL READ UNCOMMITTED; transaction 1 Select each...

Study smarter with the SolutionInn App


