A series of titrations of lactic acid, CH 3 CH(OH)COOH (pK a = 3.86) is planned. About
Question:
A series of titrations of lactic acid, CH3CH(OH)COOH (pKa = 3.86) is planned. About 1.00 mmol of the acid will be titrated with NaOH(aq) to a final volume of about 100 mL at the equivalence point.
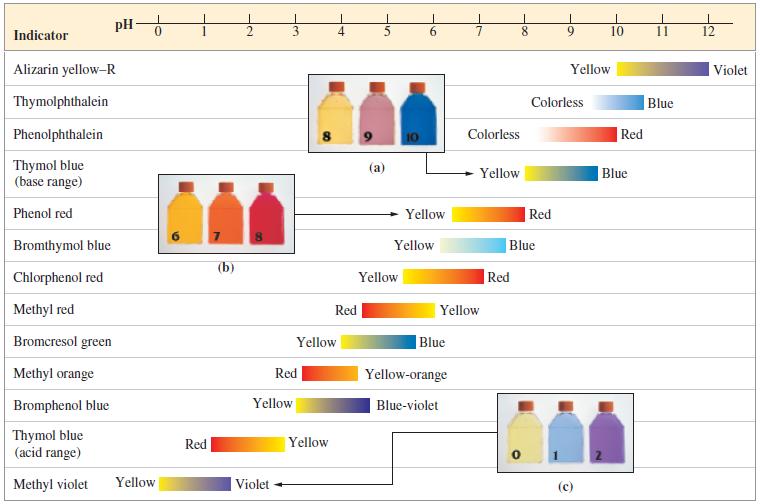
(a) Which acid–base indicator from Figure 17-7 would you select for the titration? To assist in locating the equivalence point in the titration, a buffer solution is to be prepared having the same pH as that at the equivalence point. A few drops of the indicator in this buffer will produce the color to be matched in the titrations.
(b) Which of the following combinations would be suitable for the buffer solutions: CH3COOH–CH3COO-, H2PO4-–HPO42-or NH4+–NH3?
(c) What ratio of conjugate base to acid is required in the buffer?
Figure 17-7
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette





