Question: (A) Use data from Example 15-2 to determine the value of K at 298 K for the reaction (B) For the reaction NO(g) + 1/2
(A) Use data from Example 15-2 to determine the value of K at 298 K for the reaction
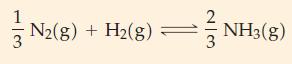
(B) For the reaction NO(g) + 1/2 O2(g) ⇌ NO2(g) at 184 °C, K = 1.2 x 102. What is the value of K at 184 °C for the reaction 2 NO2(g) ⇌ 2 NO(g) + O2(g)?
Example 15-2
At equilibrium in the following reaction at 60 °C, the partial pressures of the gases are found to be PHI = 3.70 x 10-3 bar and PH2S = 1.01 bar. What is the value of K for the reaction?
![]()
N2(g) + H(g) = NH3(g)
Step by Step Solution
3.47 Rating (157 Votes )
There are 3 Steps involved in it
To solve these problems well use the concept of the equilibrium constant K and the relationship betw... View full answer

Get step-by-step solutions from verified subject matter experts


