(a) Use the value of the van der Waals constant b for CH 4 (g), given in...
Question:
(a) Use the value of the van der Waals constant b for CH4(g), given in Table 6.5, to estimate the radius of the CH4 molecule. (See Exercise 89.) How does your estimate of the radius compare with the value r = 228 pm, obtained experimentally from an analysis of the structure of solid methane?
(b) The density of CH4(g) is 66.02 g mL-1 at 100 bar and 325 K. What is the value of the compressibility factor at this temperature and pressure?
Exercise 89
Use the value of the van der Waals constant b for He(g) given in Table 6.5, to estimate the radius, r, of a single helium atom. Give your answer in picometers.
Table 6.5
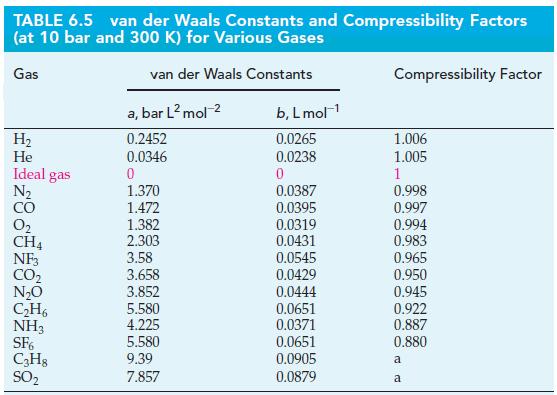
TABLE 6.5 van der Waals Constants and Compressibility Factors (at 10 bar and 300 K) for Various Gases van der Waals Constants Gas H₂ He Ideal gas N₂ CO 0₂ CH4 NF3 CO₂ N₂O C₂H6 NH3 SF6 C3H8 SO₂ a, bar L² mol-² 0.2452 0.0346 0 1.370 1.472 1.382 2.303 3.58 3.658 3.852 5.580 4.225 5.580 9.39 7.857 b, L mol-¹ 0.0265 0.0238 0 0.0387 0.0395 0.0319 0.0431 0.0545 0.0429 0.0444 0.0651 0.0371 0.0651 0.0905 0.0879 Compressibility Factor 1.006 1.005 1 0.998 0.997 0.994 0.983 0.965 0.950 0.945 0.922 0.887 0.880 a a
Step by Step Answer:
The van der Waals equation for a real gas is given by P Vb RT where P is the pressure V is the molar ...View the full answer



General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Students also viewed these Sciences questions
-
Use the value of the van der Waals constant b for He(g) given in Table 6.5, to estimate the radius, r, of a single helium atom. Give your answer in picometers. Table 6.5 TABLE 6.5 van der Waals...
-
A certain gas obeys the van der Waals equation with a =0.76 m6 Pa mol-2, its volume is found to be 4.00 X 10-4 m3 mol-1 at 288 K and 4.0 MPa. From this information calculate the van der Waals...
-
The volume of a spherical molecule can be estimated as V = b/(4N A ), where b is the van der Waals parameter for the excluded molar volume and N A is Avogadros number. Justify this relationship by...
-
If you uncover critically important information (the sort that could make or break your company) that is from a credible source and appears to be unbiased, well documented, current, and complete but...
-
In addition to your regular responsibilities, your supervisor has just assigned you to be in charge of your organizations annual golf tournament. It is expected that 100 to 150 employees will enter...
-
Schuss Inc. issued 3,000,000 of 10%, 10-year convertible bonds on April 1, 2015, at 98. The bonds were dated April 1, 2015, with interest payable April 1 and October 1. Bond discount is amortized...
-
Maribel Baltazar was hired by clothing retail merchandiser Forever 21 in 2007. During the hiring process, Baltazar was given an 11-page document to sign, two pages of which contained an arbitration...
-
Multiple Choice. Choose the best answer. 1. Which of the following fund type(s) uses the accrual basis of accounting? a. Special revenue. b. Internal service. c. Pension trust. d. Both b and c. 2....
-
1. A hollow conducting sphere has an inner and outer radius of R and R2 respectively. A point charge of +Q sits inside the hollow sphere at the center. Additionally, a charge of +2Q is distributed...
-
Assume the following initial conditions for the graphs labeled A, B, and C in Figure 6-7. (A) 10.0 mL at 400 K; (B) 20.0 mL at 400 K; (C) 40.0 mL at 400 K. Use Charless law to calculate the volume of...
-
Refer to Example 6-17. Recalculate the pressure of Cl 2 (g) by using both the ideal gas equation and the van der Waals equation at the temperatures (a) 100 C; (b) 200 C; (c) 400 C. From the results,...
-
What is the price for the April 30 Treasurybill? Maturity Mar 30 Apr 30 Jun 30 Aug 30 Days to Maturity 28 59 120 181 Bank Discount 1.20 2.00 2.45
-
#include int main () { double x = 1, y = 1; while ((((x + 1.0) x) 1.0) == 0.0) { } X * = 2.0; printf ("xu-u%. 15euu ", x); } while ((((x + y) { x) - y) != 0.0) y += 1.0; printf ("yu-u%.15 eu ", y); }...
-
4. What are the overflow and carry flags of the following operations? (Assume a four-bit system.) 1101 + 1100 1101-1100 1100 + 1010 0100-0110 0100 +0010 0100+0110 1100-0110 Carry Overflow
-
Explain the internal influences of electronic health records, what they are and how they impact organizational behavior
-
After watching the Video clips, Assess if it has an impact in both social and physical aspects of you as a student or if ever you are a businessman today as we face the covid-19 pandemic/ as we shift...
-
3- Suppose that the government is running a balanced budget and the value of purchases made by the government is 200. The consumption function is C = 200 + 0.6 Yd and planned investment is 100. b)...
-
Shoppers Market purchased for $245,000 a site on which it planned to build a new store. The site consisted of three acres of land and included an old house and two barns. County property tax records...
-
Consider the advantages and disadvantages of extending property rights so that everyone would have the right to prevent people imposing any costs on them whatsoever (or charging them to do so).
-
Explain how a non-consolidated subsidiary can be a form of off-balance-sheet financing.
-
Where can authoritative i GAAP guidance related to liabilities are found?
-
Briefly describe some of the similarities and differences between U.S. GAAP and iGAAP with respect to the accounting for liabilities.
-
Differentiate between training and developing in human resource management as to their purposes and methods. Provide an example concerning each.
-
Given data below answer the following. ID Description Predecessor Time ABCDEFGH External specifications None Review design features A Document new features A Write software A Program and test B Edit...
-
6.3-4. Consider the following problem. Minimize Z = x + 2x2, subject to and -2x + x2 1 x2x2 1 x0 x20. (a) Construct the dual problem. I (b) Use graphical analysis of the dual problem to determine...

Study smarter with the SolutionInn App


