Acetylene (C 2 H 2 ) torches are used in welding. How much heat (in kJ) evolves
Question:
Acetylene (C2H2) torches are used in welding. How much heat (in kJ) evolves when 5.0 L of C2H2 (d = 1.0967 kg/m3) is mixed with a stoichiometric amount of oxygen gas? The combustion reaction is
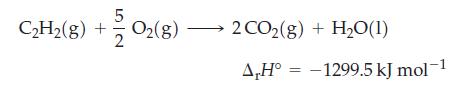
Transcribed Image Text:
5 C₂H₂(g) + +2/202(8) 2 CO2(g) + H₂O(1) A,H° -1299.5 kJ mol-1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
First lets balance the chemical equation to make sure we know how many moles of reactants and pr...View the full answer

Answered By

Sandhya Sharma
I hold M.Sc and M.Phil degrees in mathematics from CCS University, India and also have a MS degree in information management from Asian institute of technology, Bangkok, Thailand. I have worked at a international school in Bangkok as a IT teacher. Presently, I am working from home as a online Math/Statistics tutor. I have more than 10 years of online tutoring experience. My students have always excelled in their studies.
4.90+
119+ Reviews
214+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Nitric acid is used extensively for the production of inorganic and organic nitrates, for metal treatments of various kinds, and for photoengraving. It is produced by oxidizing ammonia to nitric...
-
A flow of hydrogen gas is mixed with a flow of oxygen in a stoichiometric ratio, both at 298 K and 50 kPa. The mixture burns without any heat transfer in complete combustion. Find the adiabatic flame...
-
A Claus plant converts gaseous sulfur compounds to elemental sulfur, thereby eliminating emission of sulfur into the atmosphere. The process can be especially important in the gasification of coal,...
-
A random sample of 100 students was taken from a large university to study the relationship between GPA and the number of hours of study per week. The following linear regression equation was...
-
Three inventory categories are reported on a manufacturing companys balance sheet: (a) Raw materials, (b) Goods in process, and (c) Finished goods. Identify the usual order in which these inventory...
-
An elevator is suspended from a cable. It is moving upward at a steady speed. Which is the correct free body diagram for this situation? 17- T W W B. T W T Felevator I W 13 =0 ticl E.
-
Abbott Industries is a well-known supplier of pharmaceuticals worldwide. Founded by Dr. Wallace Abbott, the company was incorporated in 1900 after he had been developing and making pharmaceuticals...
-
Levy Quilting Company makes blankets that it markets through a variety of department stores. It makes the blankets in batches of 1,000 units. Levy made 20,000 blankets during the prior accounting...
-
A 1 kg metal block is heated to 200 C and then dropped into a thermally isolated container with 4 kg of water and 100 gr of ice both at a temperature of 0 C. If the specific heat of water is cw=4186...
-
A skydiver weighs 125 pounds, and her parachute and equipment combined weigh another 35 pounds. After exiting from a plane at an altitude of 15,000 feet, she waits 15 seconds and opens her parachute....
-
The heat of neutralization of HCl(aq) by NaOH(aq) is -55.84 kJ/mol H 2 O produced. If 50.00 mL of 1.05 M NaOH is added to 25.00 mL of 1.86 M HCl, with both solutions originally at 24.72 C, what will...
-
Refer to Example 7-4. The product of the neutralization is 0.500 M NaCl. For this solution, assume a density of 1.02 g/mL and a specific heat capacity of 4.02 J g -1 C -1 . Also, assume a heat...
-
An investor enters into a short futures position in 10 contracts in gold at a futures price of $276.50 per oz. The size of one futures contract is 100 oz. The initial margin per contract is $1,500,...
-
Kohinoor Agency. operates a talent agency called Kohinoor Agency. Some clients pay in advance for services; others are billed after services have been performed. Advance payments are credited to an...
-
Navy Federal Credit Union Union's ROA is 1.17%, very similar to most publicly listed commercial banks. If you can get a ROA of 14% on Johnson and Johnson or 17% on Apple why would you ever invest in...
-
Gwen is putting aside $350/month to help save for a down payment on her first house. Assume she will get 9% per year for the entire period and will save for 12 years. How much will she have at the...
-
Assume Companies 1, 2, 3, and 4 performed equally well in Year 202X. The only differences between the companies are shown in the following table: Depreciable Life of Plant & Equipment Company 1 2 3 4...
-
An asset is acquired for a 5-year project. The book value of the asset will be worth $760,000 at the end of 5 years. The asset has an acquisition cost of $17,460,000 and will be sold for $880,000 at...
-
On November 15, TCS Company sold merchandise for $2,600 on terms of n/30 to Quaker Company. On November 20, Quaker returned some of the merchandise for a credit of $600, and on November 25, Quaker...
-
Which of the following gives the range of y = 4 - 2 -x ? (A) (- , ) (B) (- , 4) (C) [- 4, ) (D) (- , 4] (E) All reals
-
Installment Repossession Entries selected transactions of TV Land Company are presented below. 1. A television set costing $540 is sold to Jack Matre on November 1, 2010, for $900. Matre makes a down...
-
Installment-Sales Computations and Schedules Saprano Company, on January 2, 2010, entered into a contract with a manufacturing company to purchase room-size air conditioners and to sell the units on...
-
Completed-Contract Method) Monat Construction Company, Inc., entered into a firm fixed price contract with Hyatt Clinic on July 1, 2010, to construct a four-story office building. At that time, Monat...
-
How do conservation strategies and policy frameworks, such as protected areas, habitat restoration, species reintroductions, and international agreements like the Convention on Biological Diversity,...
-
A woman wants to forever endow her heir with 50,000 per year. If the woman's trust will earn 6%, how much needs to be put into the trust with these annual funds?
-
During the pandemic, why did the cargo revenue increase while passenger revenue decreased?

Study smarter with the SolutionInn App


