Assess the feasibility of the reaction by determining each of the following quantities for this reaction at
Question:
Assess the feasibility of the reaction
![]()
by determining each of the following quantities for this reaction at 25 °C.
(a) ΔrS° (The standard molar entropy of N2F4(g) is 301.2 J mol-1 K-1.)
(b) ΔrH° (Use data from Table 10.3 and F—O and N—F bond energies of 222 and 301 kJ mol-1, respectively.)
(c) ΔrG°
Is the reaction feasible? If so, is it favored at high or low temperatures?
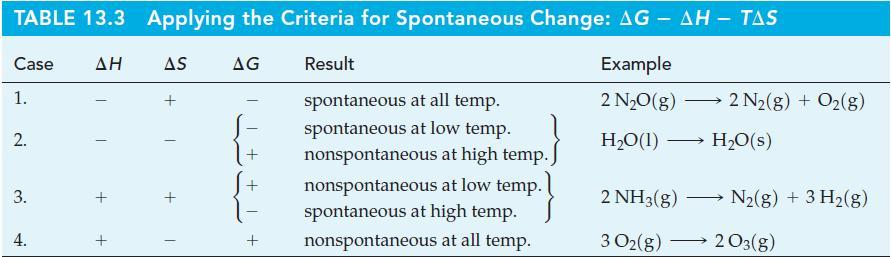
Table 10.3
Transcribed Image Text:
N₂H4(g) + 2 OF2(g) N₂F4(g) + 2 H₂O(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
To assess the feasibility of the reaction N2H4g 2 OF2g N2F4g 2H2Og at 25C well calculate the standar...View the full answer

Answered By

Aysha Ali
my name is ayesha ali. i have done my matriculation in science topics with a+ . then i got admission in the field of computer science and technology in punjab college, lahore. i have passed my final examination of college with a+ also. after that, i got admission in the biggest university of pakistan which is university of the punjab. i am studying business and information technology in my university. i always stand first in my class. i am very brilliant client. my experts always appreciate my work. my projects are very popular in my university because i always complete my work with extreme devotion. i have a great knowledge about all major science topics. science topics always remain my favorite topics. i am also a home expert. i teach many clients at my home ranging from pre-school level to university level. my clients always show excellent result. i am expert in writing essays, reports, speeches, researches and all type of projects. i also have a vast knowledge about business, marketing, cost accounting and finance. i am also expert in making presentations on powerpoint and microsoft word. if you need any sort of help in any topic, please dont hesitate to consult with me. i will provide you the best work at a very reasonable price. i am quality oriented and i have 5 year experience in the following field.
matriculation in science topics; inter in computer science; bachelors in business and information technology
_embed src=http://www.clocklink.com/clocks/0018-orange.swf?timezone=usa_albany& width=200 height=200 wmode=transparent type=application/x-shockwave-flash_
4.40+
11+ Reviews
14+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
(A) At what temperature will the formation of NO 2 (g) from NO(g) and O 2 (g) have Kp = 1.50 x 10 2 ? For the reaction 2 NO(g) + O 2 (g) 2 NO 2 (g) at 25 C, r H = -114.1 kJ mol -1 , and r S =...
-
At what temperature will the equilibrium constant for the formation of NOCl(g) be K = 1.00 x 10 3 ? Data for this reaction at 25 C are 2 NO(g) + Cl(g) 2 NOCI(g) A.G = -40.9 kJ mol- AH = -77.1 kJ mol-...
-
For parts (a) and (b) of this problem, use the following standard reduction potentials, free energies, and nonequilibrium concentrations of reactants and products: ATP = 3.10 mMPi = 5. mM 5.90ADP =...
-
Suppose that Q(x, y) is a function such that 1/Q(x, y) is continuous for all (x, y). Which of the following statements are true? (a) Q(x, y) is continuous for all (x, y). (b) Q(x, y) is continuous...
-
Mussatto Corporation produces snowboards. The following per unit cost information is available: direct materials $12; direct labor $8; variable manufacturing overhead $6; fixed manufacturing overhead...
-
Stock X has a 10% expected return, a beta coefficient of 0.9, and a 35% standard deviation of expected returns. Stock Y has a 12.5% expected return, a beta coefficient of 1.2, and a 25% standard...
-
Almetals, Inc., a Michigan company, entered into a contract with the German firm Wickeder Westfalenstahl regarding the purchase of clad metal, a specialty metal used in a variety of industries but...
-
ABC, process costing Parker Company produces mathematical and financial calculators and operas at capacity. Data related to the two products are presented here. Total manufacturing overhead costs...
-
Explain an existing healthcare initiative that address the disparities veterans face with access to mental health. Explain specific goals of the specific initiative to address the gap veterans face...
-
For each of the following reactions, write down the relationship between K and either K p or K c , as appropriate. (a) 2 SO(g) + O(g) = - 2 SO3(g) 1/11 (b) HI(g) = (c) NH4HCO3(s) I2(g) NH3(g) + CO(g)...
-
Write an equation for the combustion of one mole of benzene, C 6 H 6 (l), and determine at 298 K if the products of the combustion are (a) CO 2 (g) and H 2 O(l), and (b) CO 2 (g) and H 2 O(g)....
-
(a) Use a CAS and the concept of level curves to plot representative graphs of members of the family of solutions of the differential equation dy/dx = x(1 - x)/y(-2 + y). Experiment with different...
-
Typically, cultural factors drive the differences in business etiquette encountered during international business travel. In fact, Middle Eastern cultures exhibit significant differences in business...
-
You are preparing for a business trip to Brazil, where you will need to interact extensively with local professionals. As a result, you want to collect information about the local culture and...
-
Today, a lot of discussion centers on how much economic power, political influence, and international competitiveness the Peoples Republic of China (PRC) has achieved in the international marketplace...
-
Hotel Amazing is attempting to select the best of a group of independent projects competing for the firms fixed capital budget of $5.5 million. Management recognizes that any unused portion of this...
-
A 30 ft by 40 ft classroom with 8 ft high ceilings will have an ambient lighting target illuminance of 70 fc at a work plane that is 28 inches above the floor. It is anticipated that the ceiling...
-
Why was Kimmy Summers more successful using Facebook and Twitter to get volunteers and promote freshman move-in day at her university than she might have been using more conventional print media?
-
Write a while loop that uses an explicit iterator to accomplish the same thing as Exercise 7.3. Exercise 7.3. Write a for-each loop that calls the addInterest method on each BankAccount object in a...
-
Account analysis method. Gower, Inc., a manufacturer of plastic products, reports the following manufacturing costs and account analysis classification for the year ended December 31, 2009. Gower,...
-
Estimating a cost function, high-low method. Reisen Travel offers helicopter service from suburban towns to John F. Kennedy International Airport in New York City. Each of its 10 helicopters makes...
-
Estimating a cost function, high-low method. Laurie Daley is examining customer-service costs in the southern region of Capitol Products. Capitol Products has more than 200 separate electrical...
-
2. Give the closed form (i.e., the solution) for each of the following summations. (You do not have to show your work.) (a) n (i + n) = i=1 (b) n (i + n) = i=0
-
(a) Consider the following single-threaded processes, arrival times, and its period requirements: Process ID (PID) Arrival Time Period 1 0 6 2 2 4 3 4 3 5 6 2 a. Draw the timeline diagram for the CPU...
-
(b) Given the following set of independent periodic tasks, where the deadline interval is equal to the period: Process ID (PID) 1 Execution time 1 Period deadline 3 2 2 3 2 4 2 1 6 5 a. Why is this...

Study smarter with the SolutionInn App


