Assume that atoms are hard spheres, and use the metallic radius of 186 pm for Na to
Question:
Assume that atoms are hard spheres, and use the metallic radius of 186 pm for Na to estimate the volumes of one Na atom and of one mole of Na atoms. How does your result compare with the atomic volume found in Figure 9-1? Why is there so much disagreement between the two values?
Figure 9-1
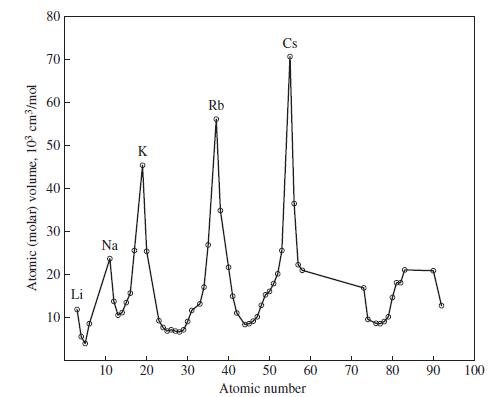
Transcribed Image Text:
Atomic (molar) volume, 103 cm³/mol 80 70 H 60 50 40 30 20 10 I T Li Na 10 K 20 30 Rb 40 50 Atomic number 60 70 80 90 100
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
To estimate the volume of one Na atom assuming that it is a hard sphere we can use the following for...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
3. Given F(x, y, z) = y.z+y.x (20 pts.) (as simplified as possible) Draw function F(x, y, z) by using NOR gates Draw function F by using NAND gates only. only. (as simplified as possible) nd the...
-
For a hydrogen atom, the probability p(r) of finding the electron within a spherical shell with inner radius r and outer radius r + dr is given by Eq. (41.7). For a hydrogen atom in the Is ground...
-
The figure illustrates how net sales of Wal-Mart Stores, Inc., have grown from 2002 through 2008. Use the midpoint formula to estimate the net sales of Wal-Mart Stores, Inc., in 2005. How does your...
-
Develop, debug, and document a program to determine the roots of a quadratic equation, ax2 + bx + c, in either a high-level language or a macro language of your choice. Use a subroutine procedure to...
-
a. Prepare a personal balance sheet for yourself as of today. Work at identifying your assets and liabilities; use rough estimates for amounts. b. Prepare a projected income statement for yourself...
-
Preparing inventory purchases budgets with different assumptions Rachel Khan has been at odds with her brother and business partner, David, since childhood. The sibling rivalry is not all bad,...
-
1. Identify an entrepreneur in your area you would like to interview. 2. Contact the person you have selected and make an appointment. Be sure to explain why you want the appointment and to give a...
-
You have been given a file that contains fields relating to CD information. Using the steps of normalization, create a logical data model that represents this file in third normal form. The fields...
-
1) For the system in the diagram m2=2.0 kg m3=3.0 kg 0=30 k=0.10 s=0.12. Find m1 and its tension for the following m3 m2 72 a) m1 accelerates upward with a=1.2 m/s^2 m1 m1= T= b) m1 moves upward with...
-
Two elements, A and B, have the electron configurations shown. (a) Which element is a metal? (b) Which element has the greater ionization energy? (c) Which element has the larger atomic radius? (d)...
-
Calculate the second ionization energy for the He atom. Compare your result with the tabulated value of 5251 kJ mol -1 .
-
In what sense would attending a Friday night college basketball game represent a hedonic- or experiential- based behavior?
-
MyManagementLab Onlycomprehensive writing assignment for this chapter.
-
Roundtable discussion: When you determine requirements for a system through a method such as an interview, you assume that the person you are interviewing and collecting information from wants the...
-
Select an information system used in your organization or in your school. Interview a systems analyst or designer who is familiar with the system. Based upon the information provided, do the...
-
The student table you are working with contains the attributes: STUDENT ID, NAME, PHONE NUMBER , and MAJOR. Normalize to 3NF.
-
A customer goes to a shoe store and purchases several pairs of shoes. Diagram this relationship.
-
Capitalizing versus expensing-effect on ROI. Early in January 2013, Tellco, Inc., acquired a new machine and incurred $300,000 of interest, installation, and overhead costs that should have been...
-
On October 31 Juanita Ortega, owner of Outback Guide Service, received a bank statement dated October 30. Juanita found the following: 1. The checkbook has a balance of $2,551.34. 2. The bank...
-
The Midwest Consulting Group (MCG) helps companies build balanced scorecards. As part of its marketing efforts, MCG conducts an annual balanced scorecard workshop for prospective clients. As MCGs...
-
The Walton Toy Company manufactures a line of dolls and a doll dress sewing kit. Demand for the dolls is increasing, and management requests assistance from you in determining an economical sales and...
-
Prentice Company is considering dropping one of its product lines. What costs of the product line would be relevant to this decision? Irrelevant?
-
Be sure that you are referencing at least 3 scholarly sources and using proper APA format and guidelines. Your submission should be 56 pages, in which you are addressing 1 topic from below and...
-
Consider that monitoring employees' web access, and restricting access to risky sites, is a best practice for most organizations. How far should monitoring go ? For instance, consider the situations...
-
Southern Democrats voting to oppose healthcare reform in 2 0 1 0 is an example of party members doing what? Southern Democrats voting to oppose healthcare reform in 2 0 1 0 is an example of party...

Study smarter with the SolutionInn App


