Assume that the packing of spherical atoms in crystalline metals is the same for Li, Na, and
Question:
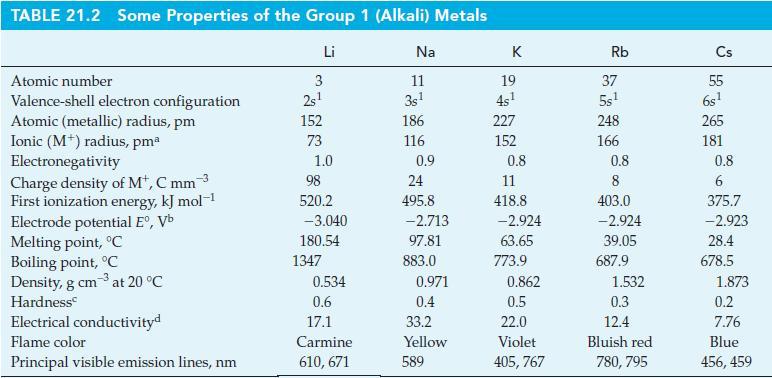
Assume that the packing of spherical atoms in crystalline metals is the same for Li, Na, and K, and explain why Na has a higher density than both Li and K. Use data from Table 21.2.
Table 21.2
Transcribed Image Text:
TABLE 21.2 Some Properties of the Group 1 (Alkali) Metals Li Atomic number Valence-shell electron configuration Atomic (metallic) radius, pm Ionic (M) radius, pmª Electronegativity Charge density of M+, C mm 3 First ionization energy, kJ mol-1 Electrode potential Eº, Vb Melting point, °C Boiling point, °C Density, g cm3 at 20 °C Hardness Electrical conductivityd Flame color Principal visible emission lines, nm 3 2s¹ 152 73 1.0 98 520.2 -3.040 180.54 1347 0.534 0.6 17.1 Carmine 610, 671 Na 11 3s¹ 186 116 0.9 24 495.8 -2.713 97.81 883.0 0.971 0.4 33.2 Yellow 589 K 19 4s¹ 227 152 0.8 11 418.8 -2.924 63.65 773.9 0.862 0.5 22.0 Violet 405, 767 Rb 37 5s¹ 248 166 0.8 8 403.0 -2.924 39.05 687.9 1.532 0.3 12.4 Bluish red 780, 795 Cs 5.5 6s¹ 265 181 0.8 6 375.7 -2.923 28.4 678.5 1.873 0.2 7.76 Blue 456, 459
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)

Answered By

OTIENO OBADO
I have a vast experience in teaching, mentoring and tutoring. I handle student concerns diligently and my academic background is undeniably aesthetic
4.30+
3+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Assume that each sequence converges and find its limit. 2,2 + 2 + 2 - 1 2 + 1' 2 2 + 2 + 1 1 2 + 1 - 2
-
In Exercises 125128, determine whether each statement is true or false. If the statement is false, make the necessary change(s) to produce a true statement. In V2 = In 2 2
-
(A) Use data from Table 19.1 to predict the probable products when Pt electrodes are used in the electrolysis of KI(aq). Table 19.1 (B) In the electrolysis of AgNO 3 (aq), what are the expected...
-
If $4000 is deposited into an account paying 3% interest compounded annually and at the same time $2000 is deposited into an account paying 5% interest compounded annually, after how long will the...
-
Using the information from PE 12-50, make the necessary journal entry to record the purchase of this held-to-maturity security.
-
What steps are required in the development of an ER diagram?
-
In the past five years, there have been significant innovations in technology such as smartphones and tablets. Technology companies rely on intellectual property (IP) rights, such as patents,...
-
Internal Indexes?Dollar-Value LIFO On January 1, 2010, Bonanza Wholesalers Inc. adopted the dollar-value LIFO inventory method for income tax and external financial reporting purposes. However,...
-
e3z (i) Find all the poles of the function f (z) = and plot them on 22(22 +22+2) (ii) an Argand diagram. Hence evaluate the integral of (2) dz, writing your solutions in the form a + jb where a and b...
-
The reaction of borax, calcium fluoride, and concentrated sulfuric acid yields sodium hydrogen sulfate, calcium sulfate, water, and boron trifluoride as products. Write a balanced equation for this...
-
The dissolution of MgCO 3 (s) in NH 4 + (aq) can be represented as Calculate the molar solubility of MgCO 3 in each of the following solutions: (a) 1.00 M NH 4 Cl(aq); (b) A buffer that is 1.00 M NH...
-
Sketch the region of integration. Then evaluate the iterated integral. 4 Jo Jy x sin x dx dy
-
how does trauma -informed practice apply to depressed elderly?
-
have a text file named students.txt that has data like the below 412 18 male drumming 446 23 female running ... (keeps on going with this structure 1.student number 2.age 3.gender 4. hobby) How could...
-
Assume Jorge Cantu earns gross pay of $1,500 during the current biweekly pay period ending January 26. Calculate Jorge's net pay based on the following assumptions: Jorge pays 20% in income tax,...
-
How to make this code generic from int to String? public class Shellsort { /* An utility function to print array of size n*/ static void printArray( int arr[]) { int n =arr.length; for ( int i=0;i...
-
Code declaration statements (including the assignment of an initial value) for the following values: Your jogging speed in miles per hour (mph). FIT1051 lecturer allocated to a workshop. The capacity...
-
Explain the distinction between a void and a voidable contract; between an executed and an executory contract; between a unilateral and a bilateral contract.
-
Baxter, Inc., owns 90 percent of Wisconsin, Inc., and 20 percent of Cleveland Company. Wisconsin, in turn, holds 60 percent of Clevelands outstanding stock. No excess amortization resulted from these...
-
Stock A has an expected return of 7%, a standard deviation of expected returns of 35%, a correlation coefficient with the market of 0.3, and a beta coefficient of !0.5. Stock B has an expected...
-
A stock had a 12% return last year, a year when the overall stock market declined. Does this mean that the stock has a negative beta and thus very little risk if held in a portfolio? Explain.
-
If investors aversion to risk increased, would the risk premium on a high-beta stock increase by more or less than that on a low-beta stock? Explain. Discuss.
-
1. How would you set up an automated answering system for a busy medical office? Make a list of options you would use as categories for patient choices. 2. Create a list of guidelines for using the...
-
Samuel is the investments director for the Metro City Police Department pension plan, which currently has a value of approximately $500 million. Recently Samuel hired a new Edwards grad named Jess...
-
How leadership can become one of the major attributes of an individual?

Study smarter with the SolutionInn App


