Question: (A) Use data from Table 19.1 to predict the probable products when Pt electrodes are used in the electrolysis of KI(aq). Table 19.1 (B) In
(A) Use data from Table 19.1 to predict the probable products when Pt electrodes are used in the electrolysis of KI(aq).
Table 19.1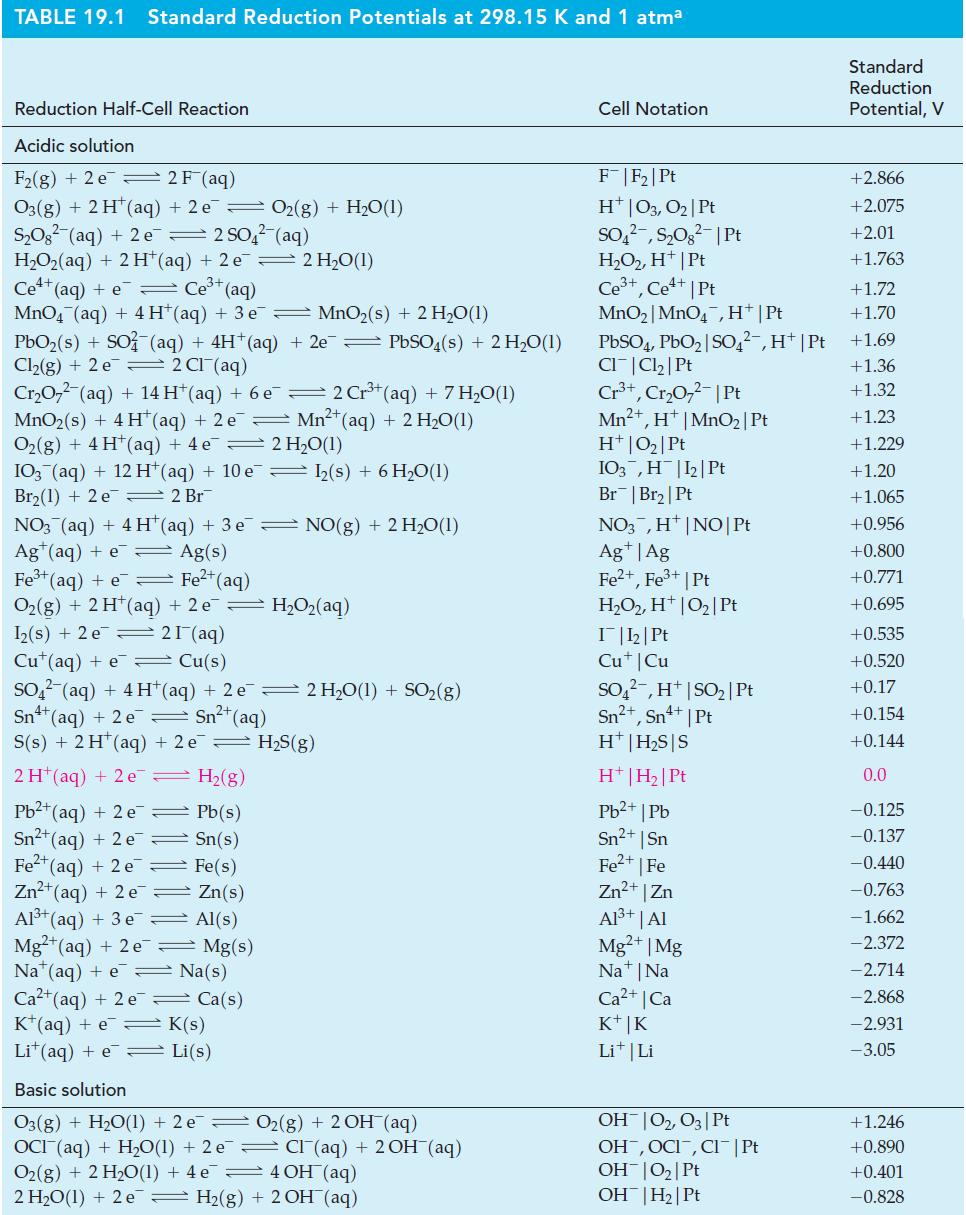
(B) In the electrolysis of AgNO3(aq), what are the expected electrolysis products if the anode is silver metal and the cathode is platinum?
TABLE 19.1 Standard Reduction Potentials at 298.15 K and 1 atma Reduction Half-Cell Reaction Acidic solution F(g) + 2 e 2 F (aq) O3(g) + 2 H+ (aq) + 2 e O(g) + HO(1) SO (aq) + 2 e 2 SO4(aq) H,Oz(aq) + 2H*(aq) +2e
Step by Step Solution
3.49 Rating (149 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


