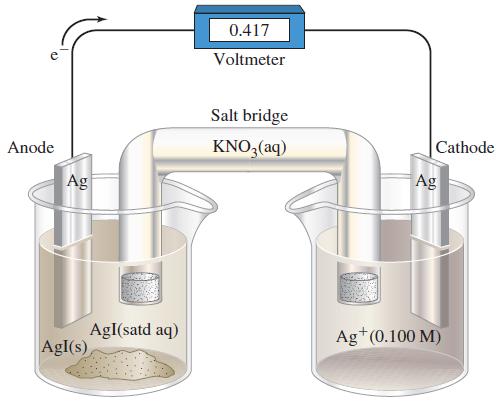
Assume that the volume of each solution in Figure 19-22 is 100.0 mL. The cell is operated
Question:
Assume that the volume of each solution in Figure 19-22 is 100.0 mL. The cell is operated as an electrolytic cell, using a current of 0.500 A. Electrolysis is stopped after 10.00 h, and the cell is allowed to function as a voltaic cell. What is Ecell at this point?
Figure 19-22
Transcribed Image Text:
Anode Ag AgI(s) AgI(satd aq) 0.417 Voltmeter Salt bridge KNO3(aq) Cathode Ag Ag+ (0.100 M)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
To answer this question we need to first calculate the amount of silver deposited on the cathode during electrolysis We can use Faradays first law of ...View the full answer

Answered By

ANUJ SHARMA
I am an Expert-Q&A in Finance and Accountancy, providing solutions for Ph.D. and Masters level finance problems.
Throughout my tutoring career, my primary focus is on the intellectual enrichment of the students.
By providing them simplified explanations, and high-quality academic material for complex concepts related to theoretical and applied fields.
I believe in assisting the students in their explorations and endeavors that push them to the new extremes.
I Solve Applied and Theoretical (Academic and Real World) related to Accounting (Management, Corporate (U.S. GAAP, SEC)) and Finance (Financial Markets both Money Market and Capital Market, Capital Rationing, Valuation, Working Capital Management, Trading FX, Commodities, Equity, Options, Index, Etc.)
0.00
0 Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The Stratton Township Park is located on a piece of property that contains two golf courses, a swimming pool, and 800 acres of woods and open spaces. Three years ago, the Stratton Park Department...
-
A box is separated by a partition into two parts of equal volume. The left side of the box contains 500 molecules of nitrogen gas; the right side contains 100 molecules of oxygen gas. The two gases...
-
EXECUTIVE SUMMARY In this report, there is an in-depth analysis of logistics revolving around a case study based on an article by Emmanuel Hassoun, a supply chain professional and Pierre Mawet, a...
-
What are the main advantages and disadvantages of using 360 degree appraisal?
-
Zanda Drug Corporation buys three chemicals that are processed to produce two types of analgesics used as ingredients for popular over-the-counter drugs. The purchased chemicals are blended for two...
-
1. What sacrifices does Costco make so that it may pay employees higher wages? 2. What are the benefits of promoting from within? What might be possible drawbacks to having a high internal promotion...
-
Develop the appropriate primary research question to be associated with this design. Develop a hypothetical research scenario that would necessitate the use of the Action Research Approach and a...
-
City Taxi Service purchased a new auto to use as a taxi on January 1, 2016, for $36,000. In addition, City paid sales tax and title fees of $1,200 for the vehicle. The taxi is expected to have a...
-
Alice wants to buy apples, beets, and carrots. An apple, a beet, and a carrot cost 16 dollars, two apples and three beets cost 23 dollars, and one apple, two beets, and three carrots cost 35 dollars....
-
You prepare 1.00 L of a buffer solution that is 1.00 M NaH 2 PO 4 and 1.00 M Na 2 HPO 4 . The solution is divided in half between the two compartments of an electrolytic cell. Both electrodes used...
-
It is sometimes possible to separate two metal ions through electrolysis. One ion is reduced to the free metal at the cathode, and the other remains in solution. In which of these cases would you...
-
1. Explain how to find the stable distribution of a regular stochastic matrix? 2. What is meant by an absorbing state of a stochastic matrix?
-
A financial analyst is attempting to assess the future dividend policy of Environmental Systems by examining its life cycle. She anticipates no payout of earnings in the form of cash dividends during...
-
The income of a resident citizen for the period: Interest income from an investment in a 10-year bond P40,000 Interest income from 5-year time deposit in Philippine bank 50,000 Interest income from...
-
Question Discuss the Main features and tax advantages of Furnished holiday lettings ?
-
Hampton Industries had $58,000 in cash at year-end 2015 and $28,000 in cash at year-end 2016. The firm invested in property, plant, and equipment totaling $290,000. Cash flow from financing...
-
Jim, the owner of Big Time Restaurant, is applying for the Main Street Small Business Tax Credit I. His restaurant has 23 employees. Jim determines the restaurant had $250,000 in gross receipts for...
-
Indicate (by abbreviation) the type of hedge each activity described below would represent. Hedge . Type FV ... Fair value hedge CF ... Cash flow hedge FC ... Foreign currency hedge N ... Would not...
-
The packaging division of a company having considered several alternative package designs for the company's new product has finally brought down their choices to two designs of which only one has to...
-
On July 1, 2010, Remington Chemical Company issued $4,000,000 face value, 10%,10-year bonds at $4,543,627.This price resulted in an 8% effective-interest rate on the bonds. Remington uses the...
-
Suppan Company sold $6,000,000, 9%, 20-year bonds on January 1, 2010. The bonds were dated January 1, 2010, and pay interest on January 1 and July 1. Suppan Company uses the straight-line method to...
-
Jinkens Corporation sold $4,000,000, 8%, 10-year bonds on January 1, 2010.The bonds were dated January 1, 2010, and pay interest on July 1 and January 1. Jinkens Corporation uses the straight-line...
-
Discuss Zynga's revenue recognition policies, i.e., how Zynga recognizes its revenue, and whether its revenue recognition policy provides decision useful information in terms of relevance and...
-
In December, Mellie's Parfum's planned to sell 800 bottles of perfume but actually sold 900 bottles. Mellie Grant, the owner of the company, is reviewing the Flexible Budget Performance Report. The...
-
On December 31, 2022, Figg Company reported net sales revenue of $155,000, gain on the sale of equipment of $2,000, rent revenue of $4,000, and rent expense of $5,000. What would be reported as other...

Study smarter with the SolutionInn App


