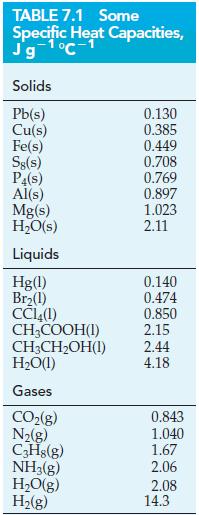
Based on specific heat capacity measurements, Pierre Dulong and Alexis Petit proposed in 1818 that the specific
Question:
Based on specific heat capacity measurements, Pierre Dulong and Alexis Petit proposed in 1818 that the specific heat capacity of an element is inversely related to its atomic weight (atomic mass). Thus, by measuring the specific heat capacity of a new element, its atomic weight could be readily established.
(a) Use data from Table 7.1 and inside the front cover to plot a straight-line graph relating atomic mass and specific heat capacity. Write the equation for this straight line.
(b) Use the measured specific heat capacity of 0.23 J g-1 °C-1 and the equation derived in part (a) to obtain an approximate value of the atomic mass of cadmium, an element discovered in 1817.
(c) To raise the temperature of 75.0 g of a particular metal by 15 °C requires 450 J of heat. What might this metal be?
Table 7.1
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette




