Benzenediazonium chloride decomposes by a first-order reaction in water, yielding N 2 (g) as one product. The
Question:
Benzenediazonium chloride decomposes by a first-order reaction in water, yielding N2(g) as one product.
![]()
The reaction can be followed by measuring the volume of N2(g) as a function of time. The data in the table on the next page were obtained for the decomposition of a 0.071 M solution at 50 °C, where t = ∞ corresponds to the completed reaction.
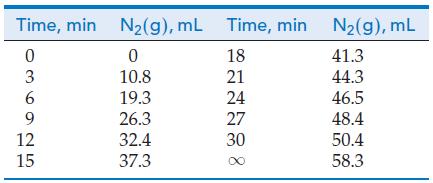
(a) Convert the information given here into a table with one column for time and the other for [C6H5N2Cl].
(b) Construct a table similar to Table 20.2, in which the time interval is Δt = 3 min.
(c) Plot graphs similar to Figure 20-2, showing both the formation of N2(g) and the disappearance of C6H5N2Cl as a function of time.
(d) From the graph of part (c), determine the rate of reaction at t = 21 min, and compare your result with the reported value of 1.1 x 10-3 M min-1.
(e) Determine the initial rate of reaction.
(f) Write the rate law for the first-order decomposition of C6H5N2Cl, and estimate a value of k based on the rate determined in parts (d) and (e).
(g) Determine t1/2 for the reaction by estimation from the graph of the rate data and by calculation.
(h) At what time would the decomposition of the sample be three-fourths complete?
(i) Plot ln[C6H5N2Cl] versus time, and show that the reaction is indeed first order.
(j) Determine k from the slope of the graph of part (i).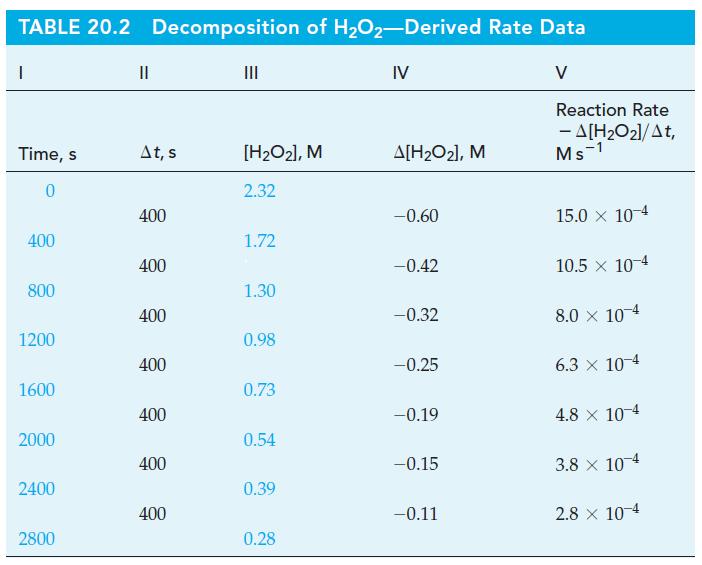
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette




