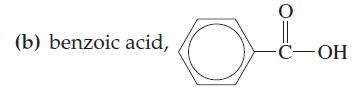
Benzoic acid, C 6 H 5 COOH, is much more soluble in NaOH(aq) than it is in
Question:
Benzoic acid, C6H5COOH, is much more soluble in NaOH(aq) than it is in pure water. Can you suggest a reason for this? The structural formula for benzoic acid is given in Exercise 5(b).
Exercise 5(b)
Two of the substances listed here are highly soluble in water, two are only slightly soluble in water, and two are insoluble in water. Indicate the situation you expect for each one.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





