Chile saltpeter is a natural source of NaNO 3 ; it also contains NaIO 3 . The
Question:
Chile saltpeter is a natural source of NaNO3; it also contains NaIO3. The NaIO3 can be used as a source of iodine. Iodine is produced from sodium iodate in a two-step process occurring under acidic conditions:
IO3-(aq) + HSO3-(aq) → I-(aq) + SO42-(aq) (not balanced)
I-(aq) + IO3-(aq) → I2(s) + H2O(l) (not balanced)
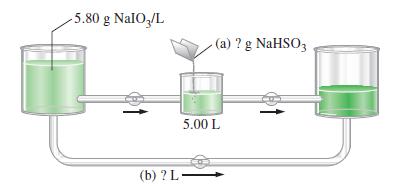
In the illustration, a 5.00 L sample of a NaIO3(aq) solution containing 5.80 g NaIO3/L is treated with the stoichiometric quantity of NaHSO3 (no excess of either reactant). Then, a further quantity of the initial NaIO3(aq) is added to the reaction mixture to bring about the second reaction.
(a) How many grams of NaHSO3 are required in the first step?
(b) What additional volume of the starting solution must be added in the second step?
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette





