Consider two cells involving two metals X and Y In the first cell electrons flow from the
Question:
Consider two cells involving two metals X and Y
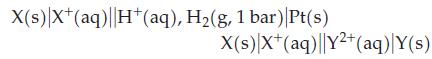
In the first cell electrons flow from the metal X to the standard hydrogen electrode. In the second cell electrons flow from metal X to metal Y. Is E°X+/X greater or less than zero? Is E°X+/X 7 EY2+/Y? Explain.
Transcribed Image Text:
X(s) X+ (aq) |H+ (aq), H₂(g, 1 bar)|Pt(s) X(s) X+ (aq)||Y²+ (aq) Y(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
In the given cells you have two halfreactions In the first cell Xs Xaq e In the second cell Xs Ys e ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
In 2008 and 2009, the US economy and many others around the world experienced a financial crisis and a severe downturn in economic activty. In many ways, it was the worst macroeconomic event in more...
-
Your mother made delicious Muffins from scratch, and she left you the recipe. Everyone raved about them. Now, you are also making Muffins just as good as moms. But, you dont want to divulge the...
-
Consider an industry where there are two firms having identical flat marginal cost curves. Price and output in the industry are determined as follows: First Firm 1 announces how much it will produce,...
-
You recently joined an accounting consulting firm. Your first client is a new manufacturing start-up that is trying to set up their costing system, and develop good processes for their upcoming...
-
On January 1, 2007, the company purchased equipment for $100,000. Originally, the equipment had a 12-year expected useful life and $4,000 residual value. The company uses straight-line depreciation....
-
Table shows supply and demand schedules for the British pound. Assume that exchange rates are flexible. a. The equilibrium exchange rate equals____. At this exchange rate, how many pounds will be...
-
Refer to the information from QS 21-18. Compute the variable overhead spending variance and the variable overhead efficiency variance and classify each as favorable or unfavorable. Data From QS 21-18...
-
You have been asked to audit Greystone Company. During the course of your audit, you are asked to prepare comparative data from the company's inception to the present. You have determined the...
-
There are 15 exceptions to carrier liability under the Carriage of Goods by Sea Act other than "perils of the sea". Which ones are similar to an event of force majeure that are usually placed in a...
-
Silver tarnish is mainly Ag 2 S: A tarnished silver spoon is placed in contact with a commercially available metallic product in a glass baking dish. Boiling water, to which some NaHCO 3 has been...
-
In 1982, the International Union of Pure and Applied Chemistry (IUPAC) redefined the standard state pressure to be 1 bar. As a result, standard reduction potentials for half-cell reactions must be...
-
A bond with a par value of $1000 has a coupon rate of 7 percent and matures in 15 years. Using a spreadsheet program, graph its price versus different yields to maturity, ranging from 1 percent to 20...
-
Choose a theory that criminal justice professionals use in one of the branches of the criminal justice system (law enforcement or corrections). Describe a real-life example of how this theory is...
-
Acme Company is considering replacing outdated production equipment that will allow for production cost savings of $20,000 per month. The new equipment will have a five-year life and cost $800,000,...
-
41) Teal Mountain Inc. earns $1308000 and pays cash dividends for $392400 during 2017. Bramble Corporation owns 74250 of the 225000 outstanding shares of Teal Mountain. How much revenue from...
-
Jim owns and operates a pizza franchise that makes and sells made to order deep-dish pizzas, Chicago style. The expected selling price is $14 per pizza. The projected variable cost per pizza is $4....
-
Barnes Company reports the following operating results for the month of August: sales $320,000 (units 5,000); variable costs $216,000; and fixed costs $70,500. Management is considering the following...
-
El Gato Painting Company maintains a checking account at American Bank. Bank statements are prepared at the end of each month. The November 30, 2011, reconciliation of the bank balance is as follows:...
-
How will relating product contribution margin s to the amount of the constrained resource they consume help a company maximize its profits?
-
Summarized operations for J. R. Ross Co. for the month of July are as follows. Revenues earned: for cash $20,000; on account $70,000. Expenses incurred: for cash $26,000; on account $40,000. Indicate...
-
Presented below is the basic accounting equation. Determine the missing amounts. Assets = Liabilities + Owners Equity (a) $90,000 $50,000 ? (b) ? $40,000 $70,000 (c) $94,000 ? $60,000
-
Given the accounting equation, answer each of the following questions (a) The liabilities of McGlone Company are $120,000 and the owners equity is $232,000.What is the amount of McGlone Companys...
-
The mass and length of the bar are m = 4 kg and /= 1.2 m. The spring constant is k = 180 N/m. If the bar is released from rest in the position 0 = 10 with the spring unstretched, what is its angular...
-
Apply resource-based view (VRIN framework) to classify resources required to pursue the opportunity. Classify resources required by informal firms. Define informal firms core competency and explain...
-
1. What type of conflict(s) existed between the two leaders? Explain your answer. 2. What did the two union leaders do to resolve their conflict? 3. In hindsight, what might have been done to resolve...

Study smarter with the SolutionInn App


