In 1982, the International Union of Pure and Applied Chemistry (IUPAC) redefined the standard state pressure to
Question:
In 1982, the International Union of Pure and Applied Chemistry (IUPAC) redefined the standard state pressure to be 1 bar. As a result, standard reduction potentials for half-cell reactions must be adjusted accordingly. In this problem, we illustrate the approach to adjusting these values. Since the hydrogen electrode reaction is assigned a value of 0 V, for both the old (1 atm) and new (1 bar) standards of pressure, the change in reduction potential for a half reaction should be calculated from the balanced equation that includes the following hydrogen electrode half-cell reaction.
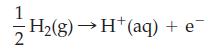
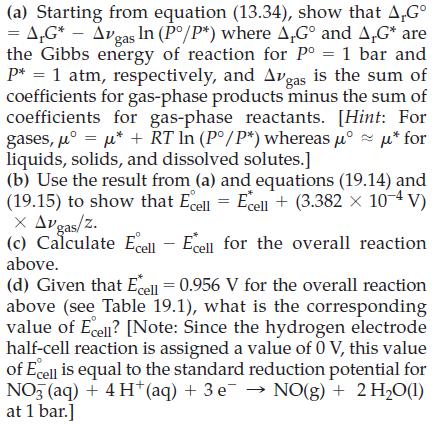
For example, to calculate the change in reduction potential for the half-cell reaction NO3‾(aq) + 4 H+(aq) + 3 e‾ → NO(g) + 2 H2O(l), we focus on the overall equation obtained by combining these two half-reactions.
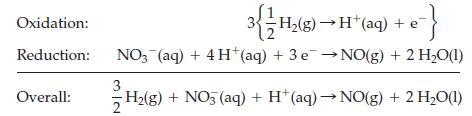
Since the cell potential is directly proportional to the Gibbs energy of reaction, ΔrG = -zFEcell, the change in cell potential that results from a change in pressure (from 1 atm to 1 bar) is obtained by first calculating the corresponding change in the Gibbs energy of reaction.
Eq. 13.34
![G = [c + dMB + ] Weighted sum of values for products [ + b + ] (13.34) Weighted sum of values for](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/0/8/0/60165646e19a31de1701080600190.jpg)
Eq. 19.14
![]()
Eq. 19.15
![]()
Table 19.1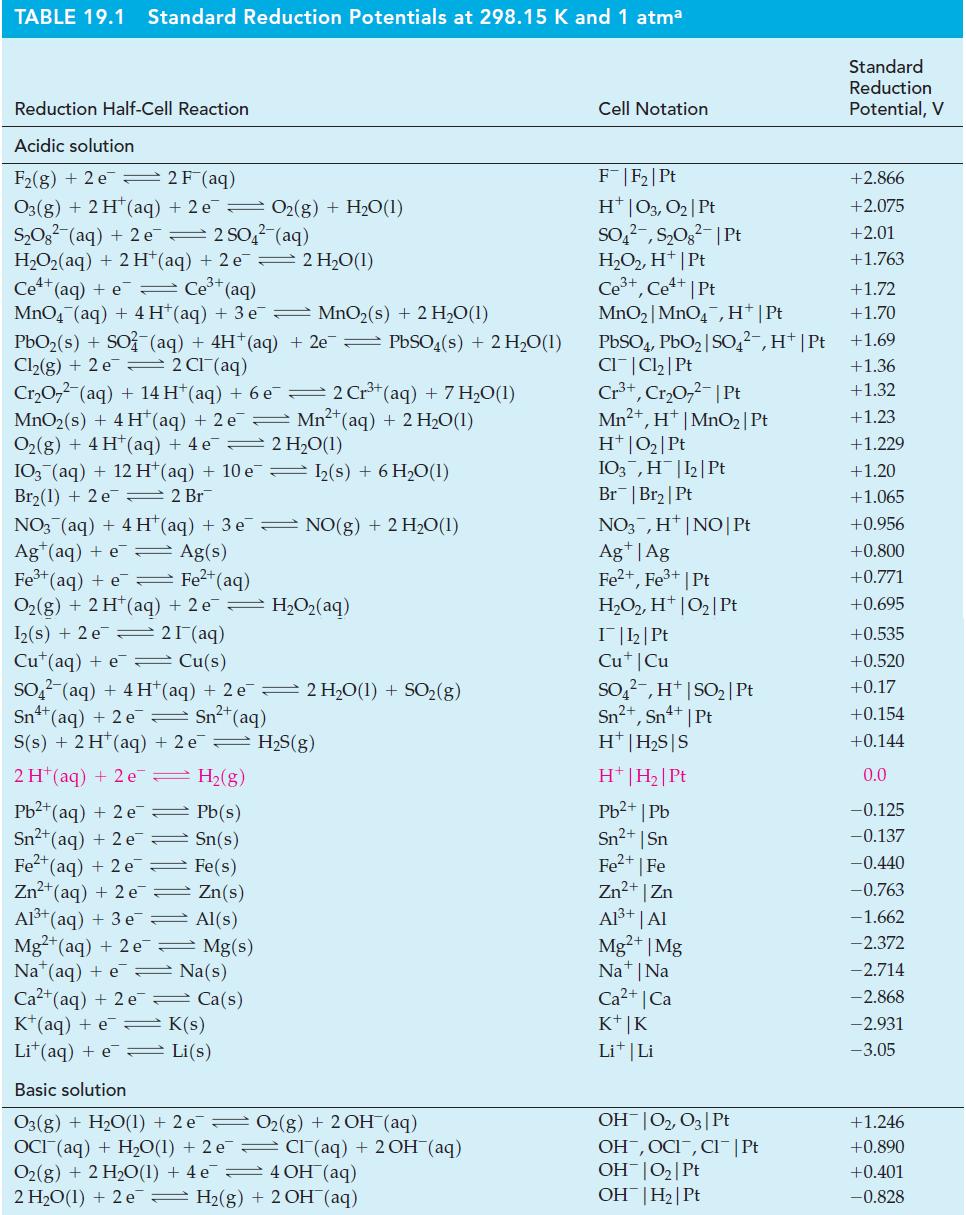
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette





