Describe a hybridization scheme for the central Cl atom in the molecule ClF 3 that is consistent
Question:
Describe a hybridization scheme for the central Cl atom in the molecule ClF3 that is consistent with the geometric shape pictured in Table 10.1. Which orbitals of the Cl atom are involved in overlaps, and which are occupied by lone-pair electrons?
Table 10.1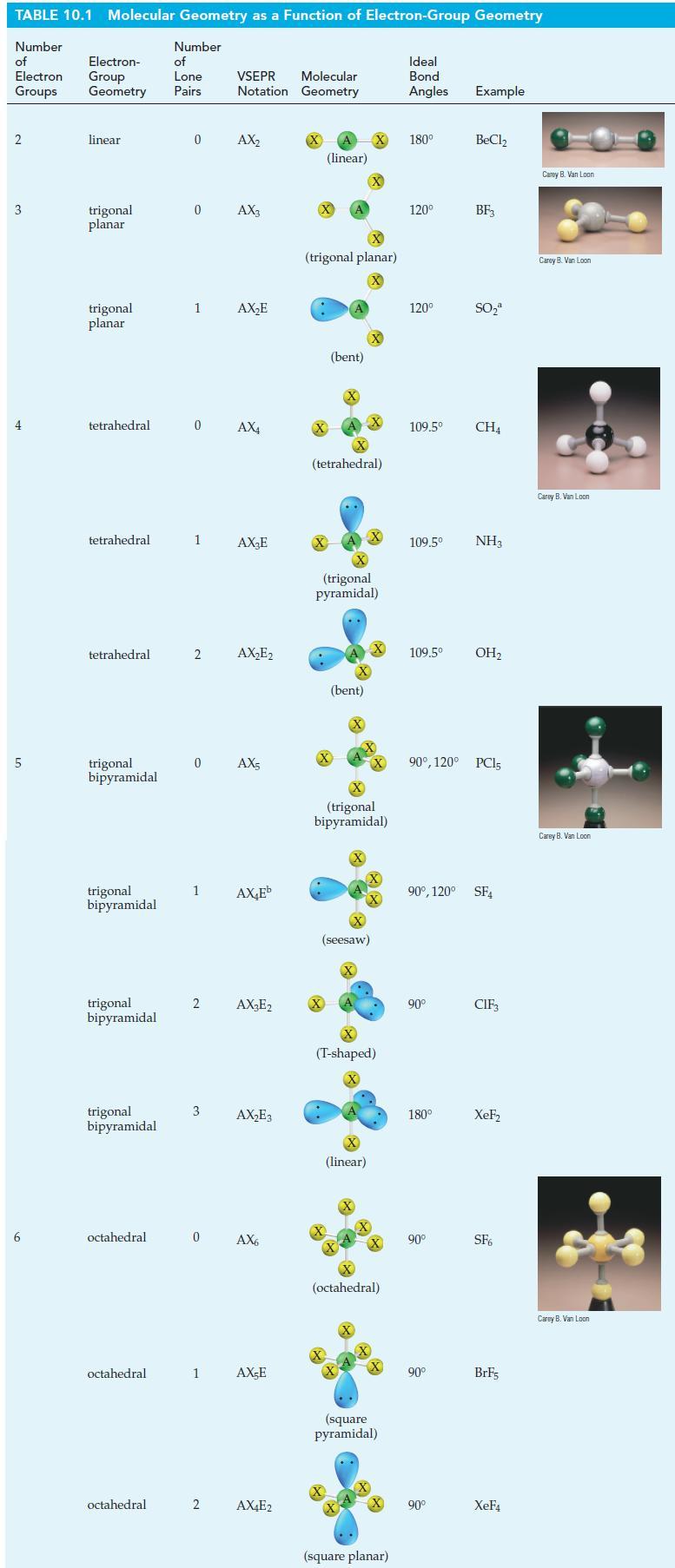
Transcribed Image Text:
TABLE 10.1 Molecular Geometry as a Function of Electron-Group Geometry Number of Electron Groups 2 3 4 5 6 Electron- Group Geometry linear trigonal planar trigonal planar tetrahedral tetrahedral tetrahedral trigonal bipyramidal trigonal bipyramidal trigonal bipyramidal trigonal bipyramidal octahedral octahedral octahedral Number of Lone Pairs 0 0 1 1 2 0 AX4 1 2 3 Molecular VSEPR Notation Geometry 0 AX₂ AX3 0 AX5 2 AX₂E AX₂E AX₂E₂ AX₂Eb AX₂E2 AX₂E3 AX6 1 AX-E AX4E2 (linear) X (trigonal planar) X X (bent) (tetrahedral) .. X (trigonal pyramidal) (bent) X X (seesaw) X X (trigonal bipyramidal) X (linear) (T-shaped) X X +4+ (octahedral) X X (square pyramidal) (square planar) Ideal Bond Angles Example 180⁰ 120⁰ 120⁰ 109.5⁰ 109.5⁰ 109.5⁰ NH3 90° 180° BeCl₂ 90° BF3 90⁰, 120° PC15 90° SO₂ 90⁰, 120° SF4 90° CH4 OH₂ CIF₁ XeF₂ SF6 BrF5 XeF4 Carey B. Van Loon Carey B. Van Loon Carey B. Van Loon Carey B. Van Loon Carey B. Van Loon
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
Fig ClF3 Explanation When we talk about the hybridization of chlorine trifluoride we have to conside...View the full answer

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Describe a hybridization scheme for the central S atom in the molecule SF 4 that is consistent with the geometric shape pictured in Table 10.1. Which orbitals of the S atom are involved in overlaps,...
-
In ozone, O3, the two oxygen atoms on the ends of the molecule are equivalent to one another. (a) What is the best choice of hybridization scheme for the atoms of ozone? (b) For one of the resonance...
-
List three specific parts of the Case Guide, Objectives and Strategy Section (See below) that you had the most difficulty understanding. Describe your current understanding of these parts. Provide...
-
ABC is a partnership owned by Angus, Black, and Campini, who share profits and losses in the ratio of 2:1:1, respectively. The account balances of the partnership at June 30, 2018, follow: ABC...
-
In a contract drawn up by Booke Company, it agreed to sell and Yermack Contracting Company agreed to buy wood shingles at $6.50. After the shingles were delivered and used, Booke Company billed...
-
A cylindrical furnace for heat-treating materials in a spacecraft environment has a 90-mm diameter and an overall length of 180 mm. Heating elements in the 135-mm-long section (1) maintain a...
-
What are the different types of consulting and litigation support activities for fraud and forensic accounting professionals?
-
Customers arrive at Rich Dunns Styling Shop at a rate of 3 per hour, distributed in a Poisson fashion. Rich can per-form haircuts at a rate of 5 per hour, distributed exponentially. a) Find the...
-
3. a) Derive D = 4x using solid state diffusion theory b) Calculate the binary O2/N2 gas diffusivity at T = 300K and compare it to the pure 02 self- diffusivity. The molecular diameter of N2 is...
-
Propose a plausible Lewis structure, geometric structure, and hybridization scheme for the ONF molecule.
-
For the NO 3 - ion, one molecular orbital in the system is bonding and one is antibonding. How many nonbonding orbitals are there? Which of the molecular orbitals are occupied? What is the...
-
The hyperbolic sine function is defined as sinh x = e x - e -x /2 a. Determine its end behavior by analyzing and b. Evaluate sinh 0. Use symmetry and part (a) to sketch a plausible graph for y = sinh...
-
Is it true that Trust is essential in any human relationship and serves as an expression of confidence in another person or group of people? This principle about human interaction has little to do...
-
Describe the forces/atmospheric conditions on a flight Envelope. a) Identify and describe the potential hazards on the aircraft and pilot arising from different manoeuvres. b) Discuss the changes of...
-
There are many factors in healthcare that contribute to cost savings, many are efficiencies within the entity, so another entity could come way under and have a much higher profit margin because...
-
What is the primary role of commercial banks? Should banks be allowed to engage in services beyond banking (insurance, for instance)?
-
write an email to my friend that's taking too much brown sugar because of his belief that Brown sugar is healthier than White sugar which is just a health myth. (must be 400 words) 1. Write an...
-
On April 29, 2013, Quality Appliances purchased equipment for $260,000. The estimated service life of the equipment is six years and the estimated residual value is $20,000. Qualitys fiscal year ends...
-
Frontland Advertising creates, plans, and handles advertising campaigns in a three-state area. Recently, Frontland had to replace an inexperienced office worker in charge of bookkeeping because of...
-
Inventory Types what are the different inventory types? How do the types differ? Why are some types said to have dependent demand whereas other types are said to have independent demand?
-
Just-in-Time Inventory if a company moves to a JIT inventory management system, what will happen to inventory turnover? What will happen to total asset turnover? What will happen to return on equity...
-
Inventory Costs if a companys inventory carrying costs are $5 million per year and its fixed order costs are $8 million per year, do you think the firm keeps too much inventory on hand or too little...
-
Determine the hardware requirements for both VM and Linux during the preparation plan, then complete the steps for installation as follows: 1. Download and install the virtualization software 2....
-
Linux Lingo: You may have noticed by now that Linux has a lot of unusual naming conventions. Some processes and features have names inspired by historical or literary context, while some are just...
-
What are multi user systems? b) Why are they successful? 2) Concerning Linux a) What programming language is Linux written in? b) How did this language influence the success of Linux?

Study smarter with the SolutionInn App


