Determine the missing values of r H in the diagram shown below. Enthalpy NO(g) + O(g)
Question:
Determine the missing values of ΔrH° in the diagram shown below.
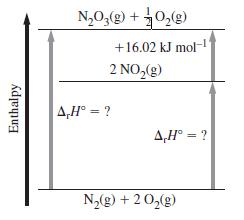
Transcribed Image Text:
Enthalpy N₂O₂(g) + O₂(g) +16.02 kJ mol-1 2 NO₂(g) A,H° = ? A,H° = ? N₂(g) + 2O₂(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
To determine the missing values of rH in the diagram we ca...View the full answer

Answered By

Kainat Shabbir
i am an experienced qualified expert with a long record of success helping clients overcome specific difficulties in information technology, business and arts greatly increasing their confidence in these topics. i am providing professional services in following concerns research papers, term papers, dissertation writing, book reports, biography writing, proofreading, editing, article critique, book review, coursework, c++, java, bootstarp, database.
5.00+
184+ Reviews
255+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The temperature interval diagram for a process is shown in Figure P15.16. For this process do the following: Compute the missing values of C p for Streams 2 and 3, the missing Q values for...
-
The data below have missing values. Determine the missing values indicated by the letters (a) through (h).
-
The data below have missing values. Determine the missing values indicated by the letters (a) to (i). Return on investment Profit margin Asset turnover Sales Investment Profit Division 6 (a) 15% 5...
-
Consider a long cylindrical solenoid with diameter R, number of current loops N and length L through which a current I runs. Now (a) Use Ampre's law to calculate the magnetic field inside the...
-
Visit your favorite fast-food restaurant. Observe its business operations. Required 1. Describe all business activities from the time a customer arrives to the time that customer departs. 2. List all...
-
Find the value of z 0.20 .
-
Gilead is a large drug producer, with a majority of its prescription drug product sales occurring in the United States. Gilead produces anti-HIV drug therapies, including the drugs Atripla, Truvada,...
-
Calls arrive at Lynn Ann Fishs hotel switchboard at a rate of 2 per minute. The average time to handle each is 20 seconds. There is only one switchboard operator at the current time. The Poisson and...
-
4. The effects of the German reunification in the Solow world. Imagine that West Germany in 1989 was well represented by the following differential equation. k =ska (8+n+g)k Let's assume that at that...
-
In this mini-case you will perform some procedures required as a part of audit planning. For ease your audit manager has already organized the workpapers and completed several of the required...
-
A handbook lists two different values for the heat of combustion of hydrogen: 33.88 kcal/g if H 2 O(l) is formed, and 28.67 kcal/g if H 2 O(g) is formed. Explain why these two values are different,...
-
The method of Exercise 98 is used in some bomb calorimetry experiments. A 1.148 g sample of benzoic acid is burned in excess O 2 (g) in a bomb immersed in 1181 g of water. The temperature of the...
-
What is application development?
-
Zero-Growth Dividend Valuation Model] Suppose a company expects to pay a $3.00 common stock dividend per share and they do not expect to grow. Thus all future dividends are expected to be $3.00 per...
-
The index value for the medical care component of the CPI is currently 451 but is expected to increase next year to 480. What is the inflation rate for medical care? Provide your answer as a...
-
Suppose you have researched the dividend payout history of a particular company and conclude that this firm grows its dividends at a constant annual rate of 3.5% (therefore, g = 0.035 in decimal...
-
Goldman Sachs, a leading investment bank based in the US, made headlines in March, 2023 with their report on Artificial Intelligence (AI). In the report, they predict that 300 million jobs worldwide...
-
The partner would like you to consider any potential planning opportunities to minimize corporate and personal tax on the sale of the shares and minimize the risk of a CRA reassessment related to the...
-
Which of the following accounts would be assigned a higher level of risk: Building or Merchandising Inventory?
-
Nike manufactures shoes and sportswear. How has the Internet changed the way this company communicates with its suppliers and retail customers?
-
Hanson Company is constructing a building. Construction began on February 1 and was completed on December 31. Expenditures were $1,800,000 on March 1, $1,200,000 on June 1, and $3,000,000 on December...
-
Hanson Company (see BE10-2) borrowed $1,000,000 on March 1 on a 5-year, 12% note to help finance construction of the building. In addition, the company had outstanding all year a 10%, 5-year,...
-
Use the information for Hanson Company from BE10-2 and BE10-3. Compute avoidable interest for Hanson Company.
-
Is Dyson cordless vacuum cleaner a radical innovation or an incremental innovation? Explain
-
4. A continuous sinusoidal wave is traveling on a string with speed 82.6 cm/s. The displacement of the particles of the string at x=9.60 cm is found to vary with time according to the equation y =...
-
A toy chest and its contents have a combined weight of W-190 N. The coefficient of static friction between toy chest and floor u is 0.440. The child in the figure attempts to move the chest across...

Study smarter with the SolutionInn App


