Dinitrogen tetroxide, N 2 O 4 (l) is an important component of rocket fuelsfor example, as an
Question:
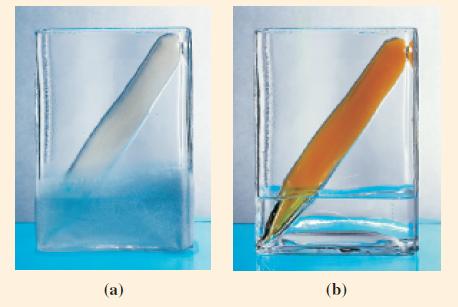
Dinitrogen tetroxide, N2O4(l) is an important component of rocket fuels—for example, as an oxidizer of liquid hydrazine in the Titan rocket. At 25 °C, N2O4 is a colorless gas that partially dissociates into NO2, a red-brown gas. The color of an equilibrium mixture of these two gases depends on their relative proportions, which in turn depends on the temperature (Fig. 15-7). Equilibrium is established in the reaction N2O4(g) ⇌ 2 NO2(g) at 25 °C. The quantities of the two gases present in a 3.00 L vessel are 7.64 g N2O4 and 1.56 g NO2. What is the value of Kc for this reaction?
Figure 15-7
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





