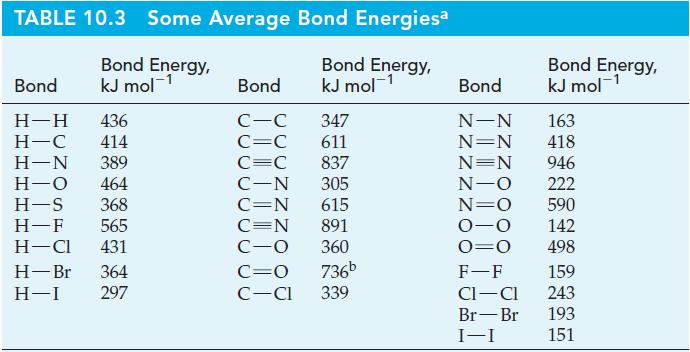
Estimate the average bond energy in O 3 (g) from the structure and data in Table 10.3.
Question:
Estimate the average bond energy in O3(g) from the structure and data in Table 10.3. Compare this result with that of Exercise 35.
Table 10.3
Exercise 35
The conversion of O2(g) to O3(g) can be accomplished in an electric discharge, 3 O2(g) → 2 O3(g). Use a bond dissociation energy of 498 kJ mol–1 for O2(g) and data from Appendix D to calculate an average oxygen-to-oxygen bond energy in O3(g).
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





