Fluoroacetic acid occurs in gifblaar, one of the most poisonous of all plants. A 0.318 M solution
Question:
Fluoroacetic acid occurs in gifblaar, one of the most poisonous of all plants. A 0.318 M solution of the acid is found to have a pH = 1.56. Calculate Ka of fluoroacetic acid.
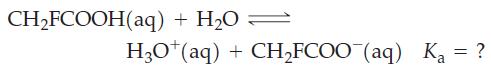
Transcribed Image Text:
CH₂FCOOH(aq) + H₂O = H3O+ (aq) + CH₂FCOO (aq) Ka = ?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (2 reviews)
To calculate the Ka of fluoroacetic acid from the given inf...View the full answer

Answered By

WAHIDUL HAQUE
hello,
I'm a professional academic solution provider working as a freelance academic solution provider since 7 years. I have completed numerous projects. Help lots of students to get good marks in their exams and quizzes. I can provide any type of academic help to your homework, classwork etc, if you are a student of Accounting, Finance, Economics, Statistics. I believe in satisfying client by my work quality, rather than making one-time profit. I charge reasonable so that we make good long term relationship. why will you choose me? i am an extremely passionate, boldly honest, ethically driven and pro-active contractor that holds each of my clients in high regards throughout all my business relations. in addition, I'll always make sure that I'm giving my 100% better in every work that will be entrusted to me to be able to produce an outcome that will meet my client's standards. so if you are a student that is now reading my profile and considering me for your academic help. please feel free to look through my working history, feedback and contact me if you see or read something that interests you. I appreciate your time and consideration.
regards
4.90+
233+ Reviews
368+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The Crazy Eddie fraud may appear smaller and gentler than the massive billion-dollar frauds exposed in recent times, such as Bernie Madoffs Ponzi scheme, frauds in the subprime mortgage market, the...
-
Googles ease of use and superior search results have propelled the search engine to its num- ber one status, ousting the early dominance of competitors such as WebCrawler and Infos- eek. Even later...
-
Read the case study "Southwest Airlines," found in Part 2 of your textbook. Review the "Guide to Case Analysis" found on pp. CA1 - CA11 of your textbook. (This guide follows the last case in the...
-
This case will enable you to practice conducting planning and substantive analytical procedures for accounts in the revenue cycle. When analyzing the financial data, you may assume that the 2015...
-
Springer Products wishes to borrow $80,000 from a local bank using its accounts receivable to secure the loan. The bank's policy is to accept as collateral any accounts that are normally paid within...
-
Reba Dixon is a fifth-grade school teacher who earned a salary of $38,000 in 2019. She is 45 years old and has been divorced for four years. She receives $1,200 of alimony payments each month from...
-
What are the two categories of data mining and knowledge discovery software?
-
The City of Green Meadows has had an employee pension fund for several years. The following is a trial balance for the fund at December 31, 2011, as well as several transactions that occurred during...
-
The 2020 season was expected to be a year of partying at Cedar Fair. Its namesake theme parkCedar Pointreached its 150 th year, and its Knotts Berry Farm was prepared to celebrate its 100 th...
-
Caproic acid, HC 6 H 11 O 2 , found in small amounts in coconut and palm oils, is used in making artificial flavors. A saturated aqueous solution of the acid contains 11 g/L and has pH = 2.94....
-
A 625 mL sample of an aqueous solution containing 0.275 mol propionic acid, CH 3 CH 2 CO 2 H, has [H 3 O + ] = 0.00239 M. What is the value of K a for propionic acid? CH3CHCOH + H0 H3O+ + CH3CHCO Ka...
-
A lone shepherd can graze 10 sheep per year in a meadow. Each additional shepherd who uses the meadow reduces the number of sheep that can be maintained by one per shepherd. If a person would rather...
-
During 2021, an historical fiction author traveled to Boston to take a summer course about Colonial history. She did so to research her next book. She spent $300 for airfare, $400 for lodging, $200...
-
Use the definite integral to find the area between the x-axis and f(x) over the indicated interval. f(x) = xe ; [0,3] The area is (Type an integer or decimal rounded to three decimal places as...
-
[2] 7. Factor the following. a) y = 10x + 20x
-
Solve by the substitution method. x - y = -6 9x + 8y = -88
-
Calculate percentage tax? Solar Sports, Inc. is a sports promoter which leases the Araneta Coliseum at a monthly rental of P900,000. It conducts boxing exhibitions and professional basketball games...
-
Do you think the interest on payday loans is too high or just right? Should Christians charge poor people interest on loans?
-
Juarez worked for Westarz Homes at construction sites for five years. Bever was a superintendent at construction sites, supervising subcontractors and moving trash from sites to landfills. He...
-
If the effect of the debit portion of an adjusting entry is to increase the balance of an asset account, which of the following statements describes the effect of the credit portion of the entry? (a)...
-
If the effect of the credit portion of an adjusting entry is to increase the balance of a liability account, which of the following statements describes the effect of the debit portion of the entry?...
-
Does every adjusting entry have an effect on determining the amount of net income for a period? Explain.
-
a) Find a vector, w of magnitude 3 that is in the opposite direction to the vector. u = (2 1, 4). = b) Let u=(-4, 3, 0) and v = (2, -1, -2) be vectors. Find the angle between u and v. Give the answer...
-
A software company is interested in improving customer satisfaction rate from the 53% currently claimed. The company sponsored a survey of 232 customers and found that 132 customers were satisfied....
-
Suppose you have a sample of scores for time on target of a tracking task. In this task, a person must follow a moving target with a mouse on a computer screen. The amount of time the person follows...

Study smarter with the SolutionInn App


