For a coordination number of four, the radius of Mn 7+ has been estimated to be 39
Question:
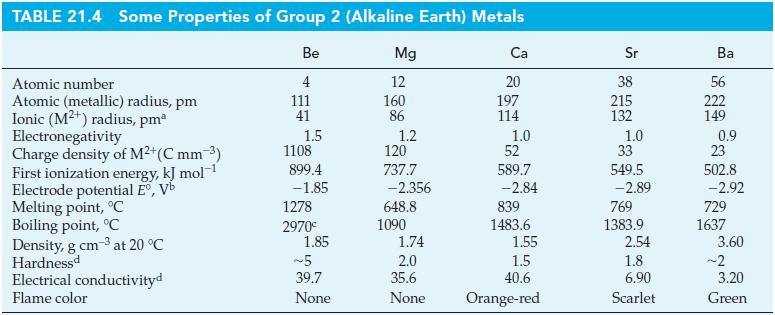
For a coordination number of four, the radius of Mn7+ has been estimated to be 39 pm. Estimate the charge density for the Mn7+ ion. Express your answer in C mm–3. How does this compare with the charge density of Be2+ given in Table 21.4? Would you expect the bonding in Mn2O7 to be primarily ionic or primarily covalent? Explain.
Table 21.4
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





