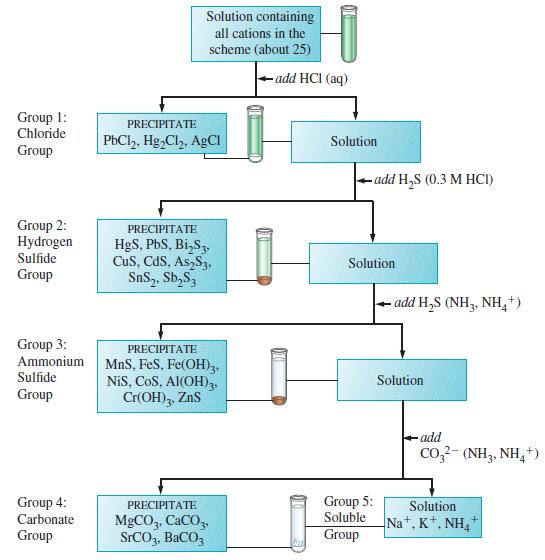
Several transition metal ions are found in cation group 3 of the qualitative analysis scheme outlined in
Question:
Several transition metal ions are found in cation group 3 of the qualitative analysis scheme outlined in Figure 18-7. At one point in the separation and testing of this group, a solution containing Fe3+, Co2+, Ni2+, Al3+, Cr3+, and Zn2+ is treated with an excess of NaOH(aq), together with H2O2(aq).
(1) The excess NaOH(aq) causes three of the cations to precipitate as hydroxides and three to form hydroxido complex ions.
(2) In the presence of H2O2(aq), the cation in one of the insoluble hydroxides is oxidized from the +2 to the +3 oxidation state, and one of the hydroxido complex ions is also oxidized.
(3) The three insoluble hydroxides are found as a dark precipitate.
(4) The solution above the precipitate has a yellow color.
(5) The dark precipitate from (3) reacts with HCl(aq), and all the cations return to solution; one of the cations is reduced from the +3 to the +2 oxidation state.
(6) The solution from (5) is treated with 6 M NH3(aq), and a precipitate containing one of the cations forms.
(a) Write equations for the reactions referred to in item (1).
(b) Write an equation for the most likely reaction in which a hydroxide precipitate is oxidized in item (2).
(c) What is the ion responsible for the yellow color of the solution in item (4)? Write an equation for its formation.
(d) Write equations for the dissolution of the precipitate and the reduction of the cation in item (5).
(e) Write an equation for the precipitate formation in item (6).
You may need solubility product and complex-ion formation data from Appendix D, together with descriptive information from this chapter and from elsewhere in the text.
Figure 18-7
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette





