For the following equilibrium reactions, calculate r G at the indicated temperature. (a) H(g) + I2(g)
Question:
For the following equilibrium reactions, calculate ΔrG° at the indicated temperature.
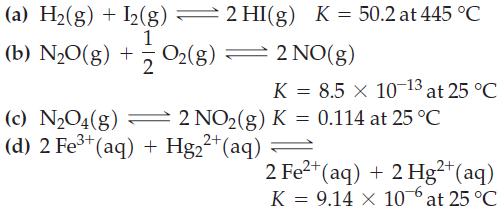
Transcribed Image Text:
(a) H₂(g) + I2(g) 2 HI(g) K = 50.2 at 445 °C 1 (b) N₂0(g) + O2(g) 2 = 2 NO(g) K = 8.5 × 10-13 at 25 °C (c) N₂O4(g) 2 NO₂(g) K = 0.114 at 25 °C (d) 2 Fe³+ (aq) + Hg₂²+ (aq) 2+ 2 Fe²+ (aq) + 2 Hg²+ (aq) K = 9.14 X 10 at 25 °C
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
From the Gibbs free energy equation Delta G 2303RTlogK T temperature in kelvin R 8314 jmole K equi...View the full answer

Answered By

Ehsan Mahmood
I’ve earned Masters Degree in Business Studies and specialized in Accounts & Finance. Couple with this, I have earned BS Sociology from renowned institute of Pakistan. Moreover, I have humongous teaching experience at Graduate and Post-graduate level to Business and humanities students along with more than 7 years of teaching experience to my foreign students Online. I’m also professional writer and write for numerous academic journals pertaining to educational institutes periodically.
4.90+
248+ Reviews
287+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
At a certain temperature the following reactions have the constants shown: Calculate the equilibrium constant Kc for the following reaction at that temperature: S(s) O2(8O2() 4.2 x 102 25(s) + 302(g)...
-
Calculate the equilibrium constant for the acid-base reactions between the following pairs of reactants. a. HCl + H2O b. CH3COOH + H2O c. CH3NH2 + H2O CH3NH3 + H20
-
Calculate the equilibrium constants of the following reactions at 25C from standard potential data: (a) Sn(s) + CuS04 (aq) ~ Cu(s) + SnS04 (aq) (b) Cu2+(aq) + Cu(s) ~ 2 Cu+{aq)
-
Prepare journal entries to record each of the following sales transactions of TFC Merchandising. TFC uses a perpetual inventory system and the gross method. May 1 9 Sold merchandise for $600, with...
-
Danner Company expects to have a cash balance of $45,000 on January 1, 2014. Relevant monthly budget data for the first 2 months of 2014 are as follows. Collections from customers: January $85,000,...
-
Determine the largest intensity w 0 of the distributed load that the beam can support if the beam can withstand a maximum bending moment of M max = 20 kN ? m and a maximum shear force of V max = 80...
-
Continuing to focus on evidence associated with the act, concealment, and conversion, use the evidentiary material to continue the examination. In addition, the examiner also starts to think of terms...
-
The current sections of Nasreen Inc.s balance sheets at December 31, 2013 and 2014, are presented here. Nasreens net income for 2014 was $153,000. Depreciation expense was $24,000. Instructions...
-
Define what is meant by an asset and a liability. Give an example of each.
-
For the reaction 2 SO 2 (g) + O 2 (g) 2 SO 3 (g), Kc = 2.8 x 10 2 M -1 at 1000 K. (a) What is r G at 1000 K? (b) If 0.40 mol SO 2 0.18 mol O 2 , and 0.72 mol SO 3 are mixed in a 2.50 L flask at...
-
At 1000 K, an equilibrium mixture in the reaction CO 2 (g) + H 2 (g) CO(g) + H 2 O(g) contains 0.276 mol H 2 0.276 mol CO 2 , 0.224 mol CO, and 0.224 mol H 2 O. (a) What is K at 1000 K? (b)...
-
Using the data in Appendix 3, calculate the standard entropy changes for the following reactions at 25C: (a) H2(g) + CuO(s) Cu(s) + H2O(g) (b) 2Al(s) + 3ZnO(s) Al2O3(s) + 3Zn(s) (c) CH4(g) + 2O2(g)...
-
Why are H&S systems essential for air travel to most U.S. cities that now have airline service?
-
A meteorologist was simulating the number of days that rain would occur in a month. The random number interval from 01 to 30 was used to indicate that rain occurred on a particular day, and the...
-
Define ASM, RASM, CASM, LF, and yield. How might a change in one affect another?
-
Which of the following is a disadvantage of simulation? a. It is inexpensive even for the most complex problem. b. It always generates the optimal solution to a problem. c. The results are usually...
-
A measure of central tendency is a. expected value. b. variance. c. standard deviation. d. all of the above.
-
Access the SEC EDGAR database (www.sec.gov) and retrieve Polaris 2011 10-K (filed February 27, 2012). Identify its auditor. What responsibility does its independent auditor claim regarding Polaris...
-
In the busy port of Chennai, India, the number of containers loaded onto ships during a 15-week period is as follows: 1. Develop a linear trend equation to forecast container loadings. 2. Using the...
-
Standard-costing method, assigning costs. Refer to the information in Exercise 17-24. Suppose Bio Dec determines standard costs of $6.60 per equivalent unit for direct materials and $10.40 per...
-
Transferred-in costs, weighted-average method. Asaya Clothing, Inc. is a manufacturer of winter clothes, It has a Knitting Department and a Finishing Department This exercise focuses on the Finishing...
-
Transferred-in costs, FIFO method. Refer to the information in Exercise 17-27. Suppose that Asaya uses the FIFO method instead of the weighted-average method in all of its departments. The only...
-
A man calculated the average value of his salary but then discovered that he had entered the wrong salary for the month of June. In fact, his income was 33,000 in June. How does the average value of...
-
One important reason for prioritizing requirements is to Minimize errors when building the system assign work within an iteration speed up the project avoid confusing the users Question 17 (1 point)...
-
Explain the concept of antigen presentation and its significance in activating T lymphocytes?

Study smarter with the SolutionInn App


