For the reaction CO(g) + H 2 O(g) CO 2 (g) + H 2 (g), K
Question:
For the reaction CO(g) + H2O(g) ⇌ CO2(g) + H2(g), Kp = 23.2 at 600 K when pressures are expressed in atmospheres. Explain which of the following situations might be found at equilibrium:
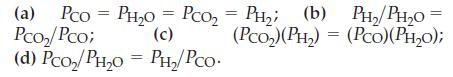
Transcribed Image Text:
(a) Pco PH₂O = PcO₂ = PH₂; (b) PH_; (b) PH,/PH,o Pco₂/Pco; (c) (PCO₂) (PH₂) = (PCO)(PH₂0); (d) Pсo₂/PH₂O = PH₂/PCO. =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
a Pco PHO PCO2 PH This situation indicates that the pressures of all the gases are equal at equilibr...View the full answer

Answered By

Shameen Tahir
The following are details of my Areas of Effectiveness. The following are details of my Areas of Effectiveness English Language Proficiency, Organization Behavior , consumer Behavior and Marketing, Communication, Applied Statistics, Research Methods , Cognitive & Affective Processes, Cognitive & Affective Processes, Data Analysis in Research, Human Resources Management ,Research Project,
Social Psychology, Personality Psychology, Introduction to Applied Areas of Psychology,
Behavioral Neurosdence , Historical and Contemporary Issues in Psychology, Measurement in Psychology, experimental Psychology,
Business Ethics Business Ethics An introduction to business studies Organization & Management Legal Environment of Business Information Systems in Organizations Operations Management Global Business Policies Industrial Organization Business Strategy Information Management and Technology Company Structure and Organizational Management Accounting & Auditing Financial Accounting Managerial Accounting Accounting for strategy implementation Financial accounting Introduction to bookkeeping and accounting Marketing Marketing Management Professional Development Strategies Business Communications Business planning Commerce & Technology Human resource management General Management Conflict management Leadership Organizational Leadership Supply Chain Management Law Corporate Strategy Creative Writing Analytical Reading & Writing Other Expertise Risk Management Entrepreneurship Management science Organizational behavior Project management Financial Analysis, Research & Companies Valuation And any kind of Excel Queries.
4.70+
16+ Reviews
34+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
A mixture of H 2 S(g) and CH 4 (g) in the mole ratio 2 : 1 was brought to equilibrium at 700 C and a total pressure of 1 atm. On analysis, the equilibrium mixture was found to contain 9.54 x 10 -3...
-
10. The role and responsibilities of the information system audit team should be established A) At the commencement of the audit. B) At every stage of the audit C) Before the audit report is drafted....
-
Sylvestor Systems borrows $110,000 cash on May 15, 2016, by signing a 60-day, 12% note. 1. On what date does this note mature? 2. Suppose the face value of the note equals $110,000, the principal of...
-
Parker Investments has EBIT of $20,000, interest expense of $3,000, and preferred dividends of $4,000. If it pays taxes at a rate of 38%, what is Parkers degree of financial leverage (DFL) at a base...
-
Yokel, a grower of soybeans, had sold soybeans to Campbell Grain and Seed Company and other grain companies in the past. Campbell entered into an oral contract with Yokel to purchase soybeans from...
-
Defendant Kenneth Blake was married to Charlene Hinton-Blake, who died in 2012. Three of Hinton-Blakes sisters, including Yvonne Hinton, assisted her with day-to-day care during a four-year period of...
-
A cost analyst for Stamper Manufacturing Co. has assembled the following data about the Model 24 stamp pad: The piece of sheet metal from which five pad cases can be made costs $0.24. This amount is...
-
A certain substance has a mass per mole of 53 g/mol. When 312 J is added as heat to a 26.0 g sample, the sample's temperature rises from 21.0C to 45.0C. What are the (a) specific heat and (b) molar...
-
Can a mixture of 2.2 mol O 2 , 3.6 mol SO 2 , and 1.8 mol SO 3 be maintained indefinitely in a 7.2 L flask at a temperature at which K c = 100 in this reaction? Explain. 2 SO2(g) + O2(g) = 2 SO3(g)
-
At 2000 K, K c = 0.154 for the reaction 2 CH 4 (g) C 2 H 2 (g) + 3 H 2 (g). If a 1.00 L equilibrium mixture at 2000 K contains 0.10 mol each of CH 4 (g) and H 2 (g), (a) What is the mole fraction of...
-
Mr. and Mrs. Napper are interested in funding their children's college education by taking out a home equity loan in the amount of $24,000. Eldridge National Bank is willing to extend a loan, using...
-
Suggest an organizational structure for marketing enterprise services, if known: The total number of employees of the manufacturing enterprise is 300 people. The company is engaged in the assembly of...
-
A 57-kg child riding a Ferris wheel (radius 10 m) travels in a vertical circle. The wheel completes one revolution every 14 s. What is the magnitude of the force on the child by the seat (i.e. normal...
-
It is required to design a pressure vessel for an operating condition of internal pressure 10 MPa and temperature 200 C. Nozzle-2 is centrally located in a 2:1 ellipsoidal head, as shown in FIGURE...
-
Find the area between f(x) and g(x) from x=2 and x=8. f(x) = -x(x-8) 10-12x g(x) = 10-
-
Suppose that a vertical well produces 2 MMscf/d of 0.71 gas-specific gravity gas through a 2 7/8 in. tubing set to the top of a gas reservoir at a depth of 10,000 ft. At tubing head, the pressure is...
-
Firm D has net income of $83,700, sales of 2,790,000, and average total assets of $1,395,000. Calculate the firms margin, turnover, and ROI.
-
If the annual fixed costs are 54,000 dinars, the occupation expense represents 20%, the contribution margin is 25%, and the unit selling price is 40 dinars. Required: Calculate the closing point of...
-
How would each of the following costs be classified if units produced is the activity base? a. Salary of factory supervisor ($70,000 per year) b. Straight-line depreciation of plant and equipment c....
-
In cost analyses, how are mixed costs treated?
-
In applying the high-low method of cost estimation, how is the total fixed cost estimated?
-
Find the equivalent capacitance of the network as shown, A 3F HH 3F HH B 3F 3F
-
r N A particle is moving along a rough circular helix as shown in the figure above. Assume that gravity (g-ge) acts opposite to the positive z-axis. Find the equations of motion.
-
4. A tire is inflated to an absolute pressure of 5 atm. As the car is driven, the temperature of the tire increases from 10C to 70C, and simultaneously, the volume of the tire increases by 5%. (a)...

Study smarter with the SolutionInn App


