For which of the following reactions would you expect the extent of the forward reaction to increase
Question:
For which of the following reactions would you expect the extent of the forward reaction to increase with increasing temperatures? Explain.
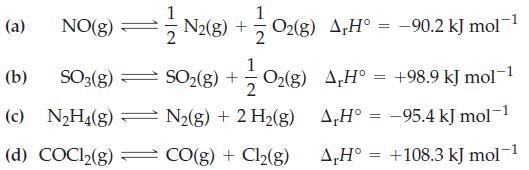
Transcribed Image Text:
(a) NO(g) =// N2(8) + O₂(8) A‚H° = −90.2 KJ mol¯ O₂(g) A,H° +98.9 kJ mol-1 A,H° = -95.4 kJ mol-1 A,Hº +108.3 kJ mol-1 (b) SO3(g) (c) N₂H4(g) (d) COC1₂(g) = SO₂(g) + 2 N₂(g) + 2 H₂(g) N₂(g) + 2 H₂(g) CO(g) + Cl₂(g) = =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 25% (4 reviews)
To determine which of the given reactions would have an increase in the extent of the forward reacti...View the full answer

Answered By

Emel Khan
I have the ability to effectively communicate and demonstrate concepts to students. Through my practical application of the subject required, I am able to provide real-world examples and clarify complex ideas. This helps students to better understand and retain the information, leading to improved performance and confidence in their abilities. Additionally, my hands-on approach allows for interactive lessons and personalized instruction, catering to the individual needs and learning styles of each student.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Which of the following reactions would you expect to have the larger rate at room temperature? Why? (Hint: Think of which would have the lower activation energy.) 2Ce4+(aq) + Hg22+(aq) 2Ce3+(aq) +...
-
KYC's stock price can go up by 15 percent every year, or down by 10 percent. Both outcomes are equally likely. The risk free rate is 5 percent, and the current stock price of KYC is 100. (a) Price a...
-
(a) In which of the following reactions would you expect the orientation factor to be least important in leading to reaction: NO + O NO2 or H + CI HCI? (b) How does the kinetic-molecular theory...
-
Transactions related to revenue and cash receipts completed by Acheville Architects Co. during the period September 2-30, 2014, are as follows: Sept. 2. Issued Invoice No. 793 to Nickle Co., $5,200....
-
What benefit is available to participants in a dividend reinvestment plan? How might the firm benefit?
-
A comparative balance sheet and income statement for Eaton Company follow: During 2008, the company sold some equipment for $18 that had cost $30 and on which there was accumulated depreciation of...
-
CGI Federal, Inc., is a corporation that provides a number of services to the United States Passport Agency, included the processing of passport application. Passport applicants must submit sensitive...
-
Martin Outdoor Furniture Company included the following stockholders equity on its year-end balance sheet at February 28, 2015: Stockholders Equity Preferred stock, 5.0% cumulativepar value $25 per...
-
need 1) ERD and 2) relational model for an airline company An airline company has many planes that travel between different airports. A trip is handled by one pilot and involves one plane. Trips...
-
The following reaction represents the binding of oxygen by the protein hemoglobin (Hb): Explain how each of the following affects the amount of Hb:O 2 : (a) Increasing the temperature; (b) Decreasing...
-
What effect does increasing the volume of the system have on the equilibrium condition in each of the following reactions? (a) C(s) + HO(g) = CO(g) + H(g) (b) Ca(OH)2(s) + CO2(g) CaCO3(s) + HO(g) (c)...
-
A chemical equation for the combustion of propane, C 3 H 8 , follows. Through this reaction is the carbon oxidized or reduced? C 3 H 8 + 5 O 2 3 CO 2 + 4 H 2 O
-
What is the definition of preoperational stage according to Jean Piaget?
-
To date, Kent has cumulative earnings of $141,000. This week Kent is paid $3,000. The total amount of Social Security tax for this week is (assume a rate of 6.2% on $142,800 for Social Security and...
-
Given: f'(x) = 4xe -1, find its antiderivative f(x), such that f(1) =6?
-
What is the sociological term denoting the process by which immigrants assimilate into the dominant cultures of the societies they have relocated to?
-
Financial s 1 . Inventory turnover ratio 2 . Average days in inventory 3 . Receivables turnover ratio 4 . Average collection period 5 . Asset turnover ratio 6 . Profit margin on sales 7 . Return on...
-
Use the information on Newell Rubbermaid shown here to answer the questions below. SHOW WORK. Current stock price $21.90 Beta 1.70 Annual Dividend $0.60 Earnings per Share $1.28 Total Shares...
-
(a) Bright Sdn Bhd (BSB) is a tax resident manufacturing company in Johor, which involves in ceramic tiles. Currently, BSBs annual sales turnover has been forecasted to be around RM 300,000 for the...
-
Give an example of how the capital expenditures budget affects other operating budgets
-
At the beginning of the period, the Fabricating Department budgeted direct labor of $22,500 and equipment depreciation of $7,000 for 900 hours of production. The department actually completed 750...
-
At the beginning of the period, the Assembly Department budgeted direct labor of $186,000 and property tax of $15,000 for 12,000 hours of production. The department actually completed 13,400 hours of...
-
Delta Supplies Corp produces a small appliance device. Its fixed cost is $3,000 and variable cost is $2.00 per unit. If Delta manufactures 1,000 units, what is the total cost of this production?
-
Find the equation of the total cost function and the average cost of a firm, if the marginal cost is MC = MC = 1.5 Q2-4 Q+12, while the total fixed cost is 20 The marginal cost of a firm is given by:...
-
Jameson has the following sources of income: Sole proprietorship income $40,000 General partnership operating income $5,000 Interest income from general partnership $1,200. What is the amount of...

Study smarter with the SolutionInn App


