What effect does increasing the volume of the system have on the equilibrium condition in each of
Question:
What effect does increasing the volume of the system have on the equilibrium condition in each of the following reactions?
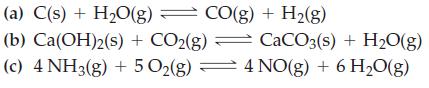
Transcribed Image Text:
(a) C(s) + H₂O(g) = CO(g) + H₂(g) (b) Ca(OH)2(s) + CO2(g) CaCO3(s) + H₂O(g) (c) 4 NH3(g) + 5 O₂(g) 4 NO(g) + 6 H₂O(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
Increasing the volume of the system has a different effect on the equilibrium condition of each reac...View the full answer

Answered By

William Otieno
I am a professional tutor and a writer with excellent skills that are important in serving the bloggers and other specialties that requires a great writer. The important aspects of being the best are that I have served so many clients with excellence
With excellent skills, I have acquired very many recommendations which have made it possible for me to survive as an excellent and cherished writer. Being an excellent content writer am also a reputable IT writer with essential skills that can make one turn papers into excellent result.
4.70+
83+ Reviews
354+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
(a) Use the reaction quotient to determine the direction the reaction must proceed to reach equilibrium. (b) Calculate the equilibrium partial pressures of the gases. (c) What effect will increasing...
-
What effect does increasing the required return have on the present value of a future amount? Why?
-
Submit the completed Marketing Math Calculations worksheet.Also, please submit a separate Word document in which you answer the following questions: If the retail price is set at $1.00, what effect...
-
Prepare journal entries for each of the following transactions: 1. Purchase equipment in exchange for cash of $22,400. 2. Provide services to customers and receive cash of $5,100. 3. Pay the current...
-
What effect did the Jobs and Growth Tax Relief Reconciliation Act of 2003 have on the taxation of corporate dividends? On corporate dividend payouts?
-
Modern Building Supply sells various building materials to retail outlets. The company has just approached Linden State Bank requesting a $300,000 loan to strengthen the Cash account and to pay...
-
Beng-Yu Woo, Xiaoming Li, and Vivian Hsiun created and patented an invention titled Full Duplex Single Chip Video Codec. At the time, Woo, Li, and Hsiun were employees of Infochips Systems, Inc....
-
The Scott Corey accounting firm is installing a new computer system. Several things must be done to make sure the system works properly before all the accounts are put into the new system. The...
-
What are the key components of a relational database management system? Why are relational database management systems different from database models that preceded the relational model?
-
For which of the following reactions would you expect the extent of the forward reaction to increase with increasing temperatures? Explain. (a) NO(g) =// N2(8) + O(8) AH = 90.2 KJ mol O(g) A,H +98.9...
-
For the reaction (a) Will K p increase, decrease, or remain constant with temperature? Explain. (b) If a constant-volume mixture at equilibrium at 298 K is heated to 400 K and equilibrium...
-
What is wrong with having tariffs and quotas? Which is the lesser of two evils, and why?
-
Safety is everybody's job and responsibility. Identify four (4) ways you can contribute to a safe workplace and remain current in relation to workplace systems, equipment and processes?
-
A local commercial bank purchased a Commercial Crime Coverage Form with Inside the Premises--Theft of Money and Securities Coverage. There is a limit of $250,000 and a deductible of $10,000. A...
-
Mary receives a base salary of $2,500 on the 1st of each month. She claims S-0. On May 1st, she received $2,500. On May 15, she received a $500 bonus. On May 29, she receives a second bonus of...
-
McAllister University, a private, nonprofit university, receives a letter from an alumnus who pledges $1,500,000 to be used for accounting research, 95% of which is expected to be collected based on...
-
Goals Project 2 Practice using loops and conditional structures Practice the use of strings
-
Response, analyze how the use of technology has impacted organizational communication both positively and negatively. How has technology impacted the verbal and nonverbal cues used in interpreting...
-
The Heese Restaurant Group manufactures the bags of frozen French fries used at its franchised restaurants. Last week, Heeses purchased and used 101,000 pounds of potatoes at a price of $ 0.70 per...
-
Soft Glow Candle Co. projected sales of 78,000 candles for 2010. The estimated January 1, 2010, inventory is 3,600 units, and the desired December 31, 2010, inventory is 4,500 units. What is the...
-
Day Timer Publishers Inc. projected sales of 205,000 schedule planners for 2010. The estimated January 1, 2010, inventory is 18,500 units, and the desired December 31, 2010, inventory is 15,000...
-
Soft Glow Candle Co, budgeted production of 78,900 candles in 2010. Wax is required to produce a candle. Assume 8 ounces (one half of a pound) of wax is required for each candle. The estimated...
-
Harry and Ginny form Potter Corporation. Harry transfers cash of $250,000 for 200 shares in Potter Corporation. Ginny transfers property with a basis of $50,000 and fair market value of $230,000. She...
-
Write formal proof justifying each step. If you use a result from class or textbook, properly cite it. Always assume n N unless stated otherwise. 1. Determine of the following series converge or not....
-
What is the distinction between a charge and a credit exchange? . How are charges applied in the accumulation bookkeeping technique? Could a charge passage at any point have a negative worth?...

Study smarter with the SolutionInn App


