Question: From data in Table 16.4, determine (a) K a for C 5 H 5 NH + ; (b) K b for HCOO - ; (c)
From data in Table 16.4, determine
(a) Ka for C5H5NH+;
(b) Kb for HCOO-;
(c) Kb for C6H5O-.
Table 16.4
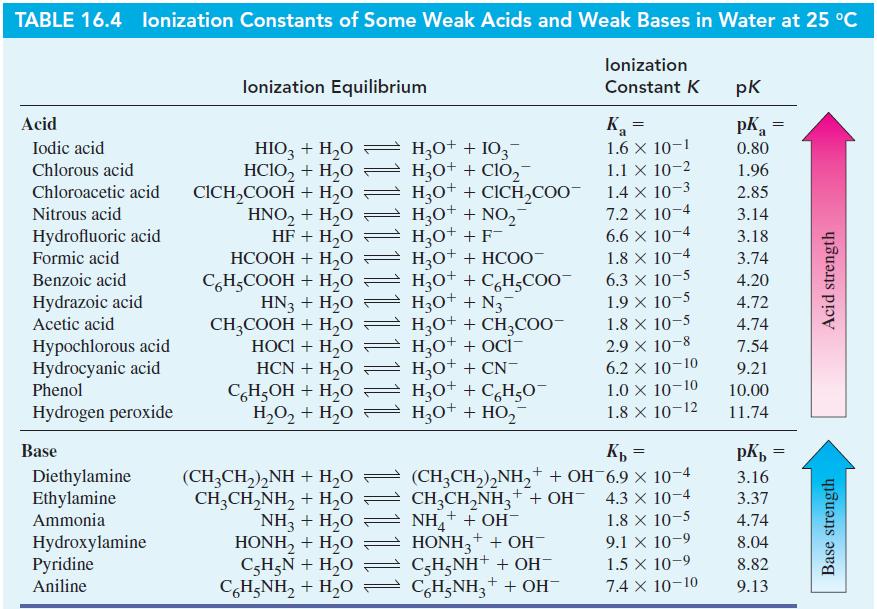
TABLE 16.4 lonization Constants of Some Weak Acids and Weak Bases in Water at 25 C lonization Constant K Acid Iodic acid Chlorous acid Chloroacetic acid Nitrous acid Hydrofluoric acid Formic acid Benzoic acid Hydrazoic acid Acetic acid Hypochlorous acid Hydrocyanic acid Phenol Hydrogen peroxide Base Diethylamine Ethylamine Ammonia Hydroxylamine Pyridine Aniline lonization Equilibrium HIO3 + HO H3O+ + 103 HCIO + HO H3O+ + ClO CICHCOOH + HO HNO + HO HF + HO HCOOH + H,O C6HCOOH + HO HN3 + HO CH3COOH + HO HOCI + HO HCN + HO C6HOH + HO H3O+ + CICHCOO H3O+ + NO HO+ + F- H,O+ + HCOO- H3O+ + C6HCOO HO + N H3O+ + CH3COO- HO+ + OCI- H3O+ + CN- H3O+ + C6HO H,O,+ H,O = H,O* + HO, HO+ (CH3CH)NH + HO CH,CH,NH, + H,O NH3 + HO HONH, + H,O C5HN + HO CH;NH, + H,O Ka 1.6 X 10-1 1.1 X 10-2 = 1.4 x 10-3 7.2 x 10-4 6.6 x 10-4 1.8 x 10-4 6.3 10-5 1.9 10-5 1.8 X 10-5 2.9 10-8 6.2 X 10-10 1.0 10-10 1.8 X 10-12 Kb = 1.8 x 10-5 (CH3CH)NH+ + OH-6.9 10-4 CH3CHNH3+ + OH- 4.3 10-4 NH+ + OH- HONH3 + + OH- C-H5NH+ + OH- C6H5NH+ + OH- 9.1 x 10-9 1.5 10-9 7.4 X 10-10 pk pk = a 0.80 1.96 2.85 3.14 3.18 3.74 4.20 4.72 4.74 7.54 9.21 10.00 11.74 pKb 3.16 3.37 4.74 8.04 8.82 9.13 = Acid strength Base strength
Step by Step Solution
3.39 Rating (152 Votes )
There are 3 Steps involved in it
a Ka for CHNH is 666x106 b Kb for HCOO is ... View full answer

Get step-by-step solutions from verified subject matter experts


