From the data in Figure 9-12, the formation of a gaseous anion Li - appears energetically favorable.
Question:
From the data in Figure 9-12, the formation of a gaseous anion Li- appears energetically favorable. That is, energy is given off when gaseous Li atoms accept electrons. Comment on the likelihood of forming a stable compound containing the Li- ion, such as Li+ Li- or Na+ Li-.
Figure 9-12
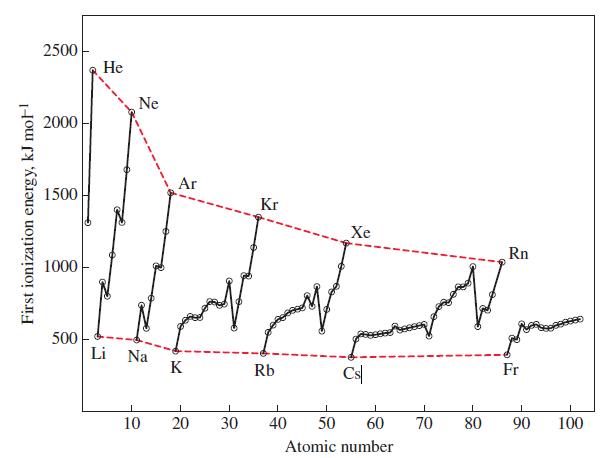
Transcribed Image Text:
First ionization energy, kJ mol-¹ 2500 2000 1500 1000 500 He Ne Li Na Ar K 10 20 30 Kr Rb Xe 1 40 Cs 1 50 60 Atomic number 70 80 Rn Fr I 90 100
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)

Answered By

Anthony Ngatia
I have three academic degrees i.e bachelors degree in Education(English & Literature),bachelors degree in business administration(entrepreneurship option),and masters degree in business administration(strategic management) in addition to a diploma in business management.I have spent much of my life in the academia where I have taught at high school,middle level colleges level and at university level.I have been an active academic essays writer since 2011 where I have worked with some of the most reputable essay companies based in Europe and in the US.I have over the years perfected my academic writing skills as a result of tackling numerous different assignments.I do not plagiarize and I maintain competitive quality in all the assignments that I handle.I am driven by strong work ethics and a firm conviction that I should "Do Unto others as I would Like them to do to me".
4.80+
76+ Reviews
152+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Estimate the As I bond length from the data in Figure 7.6, and compare your value to the experimental As I bond length in arsenic triiodide, AsI 3 , 2.55 . 1A H 2A 7A 8A 3A AA 5A 6A 0.37 0.32...
-
When a pure substance is placed in contact with water, there are three possible outcomes. The substance may do nothing that is, the substance does not dissolve and no visible change takes place. The...
-
1. Would you recommend that Roland accept the YKG Group proposal? 2. If yes, what conclusions can be drawn from the data in Phase 3 of the research? 3. If the proposal is not accepted, what...
-
Compose a detailed paper on the Emotions of employees and its impact on the organization
-
Prepare a scattergraph based on the overhead and labor-hour data in Exercise 5-26.
-
Use the sum of the first 10 terms to approximate the sum of the series. Use Exercise 34 to estimate the error.
-
T. Christian Cooper was a partner to Sanders and Richard Campbell d/b/a The Mullen Company. In 2001, Cooper helped bring about a management agreement between The Mullen Co. and Newnan Crossing...
-
Chapman Company, a major retailer of bicycles and accessories, operates several stores and is a publicly traded company. The comparative balance sheet and income statement for Chapman as of May 31,...
-
what is meant by the term functionality of a database management system? Explain the responsibility of any two functionalities of a database management system. Discuss how a database transaction is...
-
On January 1, Year 1, the Vine Company purchased 60,000 of the 80,000 ordinary shares of the Devine Company for $80 per share. On that date, Devine had ordinary shares of $3,500,000, and retained...
-
The Na + ion and the Ne atom are isoelectronic. The ease of loss of an electron by a gaseous Ne atom, first ionization energy, has a value of 2081 kJ/mol. The ease of loss of an electron from a...
-
Use ionization energies and electron affinities listed in the text to determine whether the following reaction is endothermic or exothermic. Mg(g) + 2F(g) Mg(g) + 2F (g)
-
What are the assumptions needed for the validity of the parametric test identified in Exercise 17.21 that are not needed for the KruskalWallis H test? Exercise 17.21 Identify the parametric test...
-
Standard Activity cost pool Exp. use of CD per product x Activity-Based OH rates = Cost assigned Machining 1,500 x $74 = 111,000 Setting up machines 100 x $200 = 20,000 Total cost assigned 111,00...
-
The accountant at The Lunch Lady is using a component of its organization-wide enterprise resource planning (ERP) system to prepare a payroll tax return. describe about the component of the system...
-
Sharpe contracted to sell 5 , 0 0 0 bales of cotton to Buyit. Due to drought, Sharpe's crop amounted to only 2 , 0 0 0 bales. Sharpe tendered these to Buyit. Buyit took them and sued Sharpe for...
-
obypas other Menu objects. Find the Error 1. 2. 3. .button { -fx-background-color = #0000FF; } .label { font-size: 14pt; } // This code has an error! RadioButton radio1 = new RadioButton("Option 1");...
-
Compute the amount of raw materials used during November if $ 3 6 , 0 0 0 of raw materials were purchased during the month and if the inventories were as follows: Inventories Balance November 1...
-
Peabody, Inc., sells fireworks. The companys marketing director developed the following cost of goods sold budget for April, May, June, and July. Peabody had a beginning inventory balance of $3,600...
-
From a medical tourist perspective, compare Shouldice with the traditional hospital in terms of the key factors of competition. Using Table 15-3, why would Shouldice attract patients from outside the...
-
Why might a manager prefer that budgeted rather than actual cost-allocation rates be used for costs being allocated to his or her department from another department?
-
To ensure unbiased cost allocations, fixed costs should be allocated on the basis of estimated long-run use by user-department managers. Do you agree? Why?
-
Distinguish among the three methods of allocating the costs of support departments to operating departments.
-
Markov Manufacturing recently spent $14 million to purchase some equipment used in the manufacture of disk drives. The firm expects that this equipment will have a useful life of five years, and its...
-
Carol and Ron have been married for 6 years and are experiencing a great deal of marital strain. Although they have always had conflict, it seems to have worsened in the last few months. When they...
-
Consider the penalized least square problems: N f = min{{(x, f(xi)) + 1 [* (r(m) (x)* dx] f Li=1 N - 2 a f = min | (y; f(x;)) + 2 f (f{(m +x)(x)* dx] where k is a positive integer. a Part 1: Consider...

Study smarter with the SolutionInn App


