If the pycnometer of Exercise 78 is filled with ethanol at 20 C instead of methanol, the
Question:
If the pycnometer of Exercise 78 is filled with ethanol at 20 °C instead of methanol, the observed mass is 33.470 g. What is the density of ethanol? How precisely could you determine the composition of an ethanol–methanol solution by measuring its density with a pycnometer? Assume that the density of the solution is a linear function of the volume percent composition.
Exercise 78
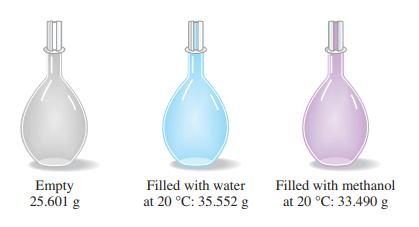
The simple device pictured here, a pycnometer, is used for precise density determinations. From the data presented, together with the fact that the density of water at 20 °C is 0.99821 g/mL, determine the density of methanol, in grams per milliliter.
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette





