In an experiment to measure K sp of CaSO 4 [D. Masterman, j. Chem. Educ., 64, 409
Question:
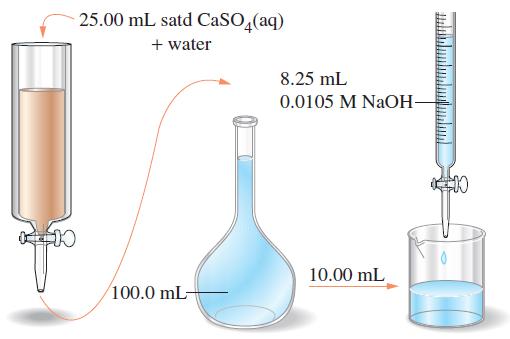
In an experiment to measure Ksp of CaSO4 [D. Masterman, j. Chem. Educ., 64, 409 (1987)], a saturated solution of CaSO4(aq) is poured into the ion-exchange column pictured. As the solution passes through the column, Ca2+ is retained by the ion-exchange medium and H3O+ is released; two H3O+ ions appear in the effluent solution for every Ca2+ ion. As the drawing suggests, a 25.00 mL sample is added to the column, and the effluent is collected and diluted to 100.0 mL in a volumetric flask. A 10.00 mL portion of the diluted solution requires 8.25 mL of 0.0105 M NaOH for its titration. Use these data to obtain a value of Ksp for CaSO4.
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette





