Question: In ionic compounds with certain metals, hydrogen exists as the hydride ion, H - . Determine the electron affinity of hydrogen; that is, r
In ionic compounds with certain metals, hydrogen exists as the hydride ion, H-. Determine the electron affinity of hydrogen; that is, ΔrH for the process H(g) + e- : H-(g). The bond energy of H2(g) from Table 10.3; -812 kJ mol-1 for the lattice energy of NaH(s); and -57 kJ mol-1 NaH for the enthalpy of formation of NaH(s).
Table 10.3
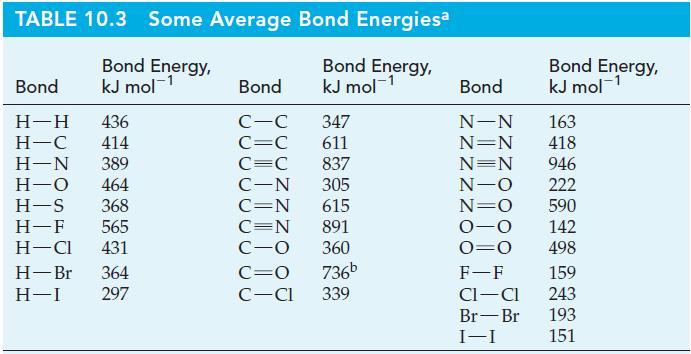
TABLE 10.3 Some Average Bond Energiesa Bond Energy, kJ mol- Bond Energy, kJ mol- Bond H-H 436 H-C 414 H-N 389 H-O 464 H-S 368 H-F 565 H-Cl 431 H-Br 364 H-I 297 Bond C-C 347 C=C 611 C=C 837 C-N 305 C=N 615 C=N 891 C-O 360 C=O 736b C-Cl 339 Bond N-N N=N N=N N-O N=O 0-0 0=0 F-F CI-CI Br-Br I-I Bond Energy, kJ mol- 163 418 946 222 590 142 498 159 243 193 151
Step by Step Solution
3.45 Rating (161 Votes )
There are 3 Steps involved in it
To determine the electron affinity of hydrogen we can use the following ... View full answer

Get step-by-step solutions from verified subject matter experts


