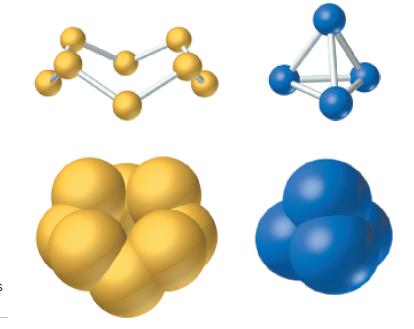
In white phosphorus, P atoms are joined into P 4 molecules (see Figure 3-5). White phosphorus is
Question:
In white phosphorus, P atoms are joined into P4 molecules (see Figure 3-5). White phosphorus is commonly supplied in chalk-like cylindrical form. Its density is 1.823 g/cm3. For a cylinder of white phosphorus 6.50 cm long and 1.22 cm in diameter, determine
(a) The number of moles of P4 present;
(b) The total number of P atoms.
Figure 3-5
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





